Real-world treatment patterns and outcomes in patients with primary hemophagocytic lymphohistiocytosis treated with emapalumab
- PMID: 38429096
- PMCID: PMC11117018
- DOI: 10.1182/bloodadvances.2023012217
Real-world treatment patterns and outcomes in patients with primary hemophagocytic lymphohistiocytosis treated with emapalumab
Abstract
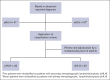
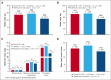
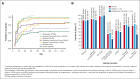
Hemophagocytic lymphohistiocytosis (HLH) is a rare, life-threatening, hyperinflammatory syndrome. Emapalumab, a fully human monoclonal antibody that neutralizes the proinflammatory cytokine interferon gamma, is approved in the United States to treat primary HLH (pHLH) in patients with refractory, recurrent, or progressive disease, or intolerance with conventional HLH treatments. REAL-HLH, a retrospective study, conducted across 33 US hospitals, evaluated real-world treatment patterns and outcomes in patients treated with ≥1 dose of emapalumab between 20 November 2018 and 31 October 2021. In total, 46 patients met the pHLH classification criteria. Median age at diagnosis was 1.0 year (range, 0.3-21.0). Emapalumab was initiated for treating refractory (19/46), recurrent (14/46), or progressive (7/46) pHLH. At initiation, 15 of 46 patients were in the intensive care unit, and 35 of 46 had received prior HLH-related therapies. Emapalumab treatment resulted in normalization of key laboratory parameters, including chemokine ligand 9 (24/33, 72.7%), ferritin (20/45, 44.4%), fibrinogen (37/38, 97.4%), platelets (39/46, 84.8%), and absolute neutrophil count (40/45, 88.9%). Forty-two (91.3%) patients were considered eligible for transplant. Pretransplant survival was 38 of 42 (90.5%). Thirty-one (73.8%) transplant-eligible patients proceeded to transplant, and 23 of 31 (74.2%) of those who received transplant were alive at the end of the follow-up period. Twelve-month survival probability from emapalumab initiation for the entire cohort (N = 46) was 73.1%. There were no discontinuations because of adverse events. In conclusion, results from the REAL-HLH study, which describes treatment patterns, effectiveness, and outcomes in patients with pHLH treated with emapalumab in real-world settings, are consistent with the emapalumab pivotal phase 2/3 pHLH trial.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: C.E.A. reports advisory board membership with Sobi Inc and Opna, and research support from Genentech and Opna. M.M.H. serves on a speaker/advisory board for Sobi Inc and is a speaker for ClearPoint. M.B.J. reports advisory board membership with Sobi Inc and received research support from Sobi Inc and Bristol Myers Squibb. J.W.L. serves on a speaker/advisory board for Sobi Inc; serves on the advisory board of Horizon; is an employee of bluebird bio; and holds stock in bluebird bio. A.O. is an employee of Sobi Inc and holds stock in Sobi Inc. P.P. is an employee of PRECISIONheor and served as consultant for Sobi Inc at the time of study. S.A.P. serves on a speaker/advisory board for Sobi Inc. A.K.R. is a speaker for BTC International Inc and Sobi Inc. J.D.Y. was an employee, at time of study, of Sobi Inc. A.Z.-L. serves as consultant for Sobi Inc. The remaining authors declare no competing financial interests.
A complete list of the members of the REAL-HLH Investigators appears in “Appendix.”
Figures



References
-
- Allen CE, McClain KL. Pathophysiology and epidemiology of hemophagocytic lymphohistiocytosis. Hematology Am Soc Hematol Educ Program. 2015;2015:177–182. - PubMed
-
- Henter JI, Elinder G, Söder O, Ost A. Incidence in Sweden and clinical features of familial hemophagocytic lymphohistiocytosis. Acta Paediatr Scand. 1991;80(4):428–435. - PubMed

