A phase I/IIa safety and efficacy trial of intratympanic gamma-secretase inhibitor as a regenerative drug treatment for sensorineural hearing loss
- PMID: 38429256
- PMCID: PMC10907343
- DOI: 10.1038/s41467-024-45784-0
A phase I/IIa safety and efficacy trial of intratympanic gamma-secretase inhibitor as a regenerative drug treatment for sensorineural hearing loss
Abstract
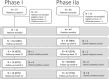
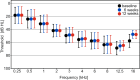
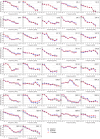
Inhibition of Notch signalling with a gamma-secretase inhibitor (GSI) induces mammalian hair cell regeneration and partial hearing restoration. In this proof-of-concept Phase I/IIa multiple-ascending dose open-label trial (ISRCTN59733689), adults with mild-moderate sensorineural hearing loss received 3 intratympanic injections of GSI LY3056480, in 1 ear over 2 weeks. Phase I primary outcome was safety and tolerability. Phase lla primary outcome was change from baseline to 12 weeks in average pure-tone air conduction threshold across 2,4,8 kHz. Secondary outcomes included this outcome at 6 weeks and change from baseline to 6 and 12 weeks in pure-tone thresholds at individual frequencies, speech reception thresholds (SRTs), Distortion Product Otoacoustic Emissions (DPOAE) amplitudes, Signal to Noise Ratios (SNRs) and distribution of categories normal, present-abnormal, absent and Hearing Handicap Inventory for Adults/Elderly (HHIA/E). In Phase I (N = 15, 1 site) there were no severe nor serious adverse events. In Phase IIa (N = 44, 3 sites) the average pure-tone threshold across 2,4,8 kHz did not change from baseline to 6 and 12 weeks (estimated change -0.87 dB; 95% CI -2.37 to 0.63; P = 0.252 and -0.46 dB; 95% CI -1.94 to 1.03; P = 0.545, respectively), nor did the means of secondary measures. DPOAE amplitudes, SNRs and distribution of categories did not change from baseline to 6 and 12 weeks, nor did SRTs and HHIA/E scores. Intratympanic delivery of LY3056480 is safe and well-tolerated; the trial's primary endpoint was not met.
© 2024. The Author(s).
Conflict of interest statement
A.G.M. Schilder advises hearing industry on clinical trial design and delivery. A.S.B. Edge is a consultant and shareholder of Audion Therapeutics BV. L.M. Middelink is consultant to Audion Therapeutics BV. The remaining authors declare no competing interests
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

