The effects of delayed annealing on the luminescent activity of heavy metal cadmium zinc phosphate glasses activated by: Er3+ and Tb3+ ions
- PMID: 38429313
- PMCID: PMC10907366
- DOI: 10.1038/s41598-024-55409-7
The effects of delayed annealing on the luminescent activity of heavy metal cadmium zinc phosphate glasses activated by: Er3+ and Tb3+ ions
Abstract
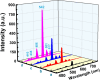
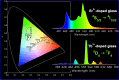
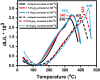
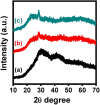
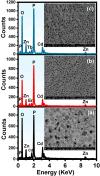
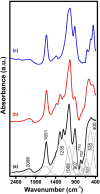
The luminescent spectra of the RE2O3-doped P2O5-CdO-ZnO glasses (RE = Er, and Tb) were investigated to separate the effects of two studied rare-earth elements and the annealing regime on the emission performance of the prepared glasses. The glasses undergo a series of collective measurements including UV-visible absorption, luminescence, thermal expansion, XRD, TEM, and FTIR. The optical UV-visible spectra of the two doped glasses reveal a UV band due to undoped glass beside and extra extended 11 peaks with the Er3+ ions with high distinct features while the Tb3+ ions samples exhibit peaks within the visible region. These peaks are correlated with transitions from the ground state in each case to specific energy transitions. The overall optical data indicate that the two rare earth ions are present in a stable trivalent state. Under UV excitation, both Er3+ and Tb3+ emit a characteristic green light corresponding to 4S3/2 → 4I15/2 and 5D4 → 7F5 transitions, respectively. The performance of the green light was identified to be enhanced by increasing the concentration of rare earth and the effect of annealing temperature. Moreover, the intensity of the infrared emission of Er3+ at 1532 nm corresponds to the (4I13/2 → 4I15/2) transition which is assumed to be developed with the effect of heating. The resultant IR spectra show distinct vibrational peaks due to phosphate groups that undergo only minor modifications when doped with rare earth elements or over-annealed.
Keywords: Annealing; Glass; Heavy metal oxide; Luminescence; Phosphate; Rare-Earth.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Brow RK. Review: The structure of simple phosphate glasses. J. Non-Cryst. Solids. 2000;263–264:1–28. doi: 10.1016/S0022-3093(99)00620-1. - DOI
-
- Smith CE, Brow RK. The properties and structure of zinc magnesium phosphate glasses. J. Non-Cryst. Solids. 2014;390:51–58. doi: 10.1016/j.jnoncrysol.2014.02.010. - DOI
-
- Shelby JE. Introduction to Glass Science and Technology, 2nd Edition. The Royal Society of Chemistry; 2005. p. 146.
-
- Musgraves JD, Hu J, Calvez L. Springer Handbook of Glass. Springer Nature; 2019. pp. 553–587.
-
- Day DE, Wu Z, Ray CS, Hrma P. Chemically durable iron phosphate glass waste forms. J. Non-Cryst. Solids. 1998;241(1):1–12. doi: 10.1016/S0022-3093(98)00759-5. - DOI
LinkOut - more resources
Full Text Sources

