Development of a deep learning model to distinguish the cause of optic disc atrophy using retinal fundus photography
- PMID: 38429319
- PMCID: PMC10907364
- DOI: 10.1038/s41598-024-55054-0
Development of a deep learning model to distinguish the cause of optic disc atrophy using retinal fundus photography
Abstract
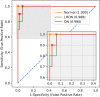
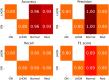
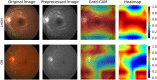
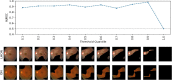
The differential diagnosis for optic atrophy can be challenging and requires expensive, time-consuming ancillary testing to determine the cause. While Leber's hereditary optic neuropathy (LHON) and optic neuritis (ON) are both clinically significant causes for optic atrophy, both relatively rare in the general population, contributing to limitations in obtaining large imaging datasets. This study therefore aims to develop a deep learning (DL) model based on small datasets that could distinguish the cause of optic disc atrophy using only fundus photography. We retrospectively reviewed fundus photographs of 120 normal eyes, 30 eyes (15 patients) with genetically-confirmed LHON, and 30 eyes (26 patients) with ON. Images were split into a training dataset and a test dataset and used for model training with ResNet-18. To visualize the critical regions in retinal photographs that are highly associated with disease prediction, Gradient-Weighted Class Activation Map (Grad-CAM) was used to generate image-level attention heat maps and to enhance the interpretability of the DL system. In the 3-class classification of normal, LHON, and ON, the area under the receiver operating characteristic curve (AUROC) was 1.0 for normal, 0.988 for LHON, and 0.990 for ON, clearly differentiating each class from the others with an overall total accuracy of 0.93. Specifically, when distinguishing between normal and disease cases, the precision, recall, and F1 scores were perfect at 1.0. Furthermore, in the differentiation of LHON from other conditions, ON from others, and between LHON and ON, we consistently observed precision, recall, and F1 scores of 0.8. The model performance was maintained until only 10% of the pixel values of the image, identified as important by Grad-CAM, were preserved and the rest were masked, followed by retraining and evaluation.
Keywords: Deep learning; Fundus photography; Leber hereditary optic neuropathy; Optic neuritis; Optic neuropathy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Computer-aided recognition of myopic tilted optic disc using deep learning algorithms in fundus photography.BMC Ophthalmol. 2020 Oct 9;20(1):407. doi: 10.1186/s12886-020-01657-w. BMC Ophthalmol. 2020. PMID: 33036582 Free PMC article.
-
Optic disc excavation in the atrophic stage of Leber's hereditary optic neuropathy: comparison with normal tension glaucoma.Graefes Arch Clin Exp Ophthalmol. 2003 Feb;241(2):75-80. doi: 10.1007/s00417-002-0598-0. Epub 2003 Jan 25. Graefes Arch Clin Exp Ophthalmol. 2003. PMID: 12605258
-
Efficacy for Differentiating Nonglaucomatous Versus Glaucomatous Optic Neuropathy Using Deep Learning Systems.Am J Ophthalmol. 2020 Aug;216:140-146. doi: 10.1016/j.ajo.2020.03.035. Epub 2020 Apr 2. Am J Ophthalmol. 2020. PMID: 32247778
-
Emerging model systems and treatment approaches for Leber's hereditary optic neuropathy: Challenges and opportunities.Biochim Biophys Acta Mol Basis Dis. 2020 Jun 1;1866(6):165743. doi: 10.1016/j.bbadis.2020.165743. Epub 2020 Feb 24. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32105823 Free PMC article. Review.
-
Investigating Leber's hereditary optic neuropathy: Cell models and future perspectives.Mitochondrion. 2017 Jan;32:19-26. doi: 10.1016/j.mito.2016.11.006. Epub 2016 Nov 12. Mitochondrion. 2017. PMID: 27847334 Review.
Cited by
-
Detection of optic disc in human retinal images utilizing the Bitterling Fish Optimization (BFO) algorithm.Sci Rep. 2024 Oct 28;14(1):25824. doi: 10.1038/s41598-024-76134-1. Sci Rep. 2024. PMID: 39468169 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

