Cancer-associated fibroblast-derived acetate promotes pancreatic cancer development by altering polyamine metabolism via the ACSS2-SP1-SAT1 axis
- PMID: 38429478
- PMCID: PMC11021164
- DOI: 10.1038/s41556-024-01372-4
Cancer-associated fibroblast-derived acetate promotes pancreatic cancer development by altering polyamine metabolism via the ACSS2-SP1-SAT1 axis
Erratum in
-
Author Correction: Cancer-associated fibroblast-derived acetate promotes pancreatic cancer development by altering polyamine metabolism via the ACSS2-SP1-SAT1 axis.Nat Cell Biol. 2024 May;26(5):840. doi: 10.1038/s41556-024-01417-8. Nat Cell Biol. 2024. PMID: 38641662 Free PMC article. No abstract available.
Abstract
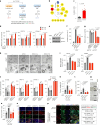
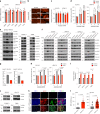
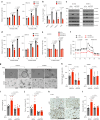
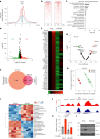
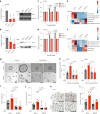
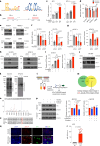
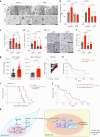
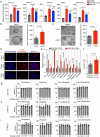
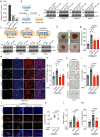
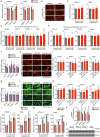
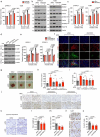
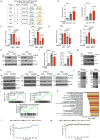
The ability of tumour cells to thrive in harsh microenvironments depends on the utilization of nutrients available in the milieu. Here we show that pancreatic cancer-associated fibroblasts (CAFs) regulate tumour cell metabolism through the secretion of acetate, which can be blocked by silencing ATP citrate lyase (ACLY) in CAFs. We further show that acetyl-CoA synthetase short-chain family member 2 (ACSS2) channels the exogenous acetate to regulate the dynamic cancer epigenome and transcriptome, thereby facilitating cancer cell survival in an acidic microenvironment. Comparative H3K27ac ChIP-seq and RNA-seq analyses revealed alterations in polyamine homeostasis through regulation of SAT1 gene expression and enrichment of the SP1-responsive signature. We identified acetate/ACSS2-mediated acetylation of SP1 at the lysine 19 residue that increased SP1 protein stability and transcriptional activity. Genetic or pharmacologic inhibition of the ACSS2-SP1-SAT1 axis diminished the tumour burden in mouse models. These results reveal that the metabolic flexibility imparted by the stroma-derived acetate enabled cancer cell survival under acidosis via the ACSS2-SP1-SAT1 axis.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- P20 GM121316/GM/NIGMS NIH HHS/United States
- U54 CA274329/CA/NCI NIH HHS/United States
- P30 CA225520/CA/NCI NIH HHS/United States
- R01 CA163649/CA/NCI NIH HHS/United States
- R37 CA276924/CA/NCI NIH HHS/United States
- T32 CA009476/CA/NCI NIH HHS/United States
- P20 GM103447/GM/NIGMS NIH HHS/United States
- P20 GM113126/GM/NIGMS NIH HHS/United States
- P50 CA127297/CA/NCI NIH HHS/United States
- R50 CA211462/CA/NCI NIH HHS/United States
- R01 CA256911/CA/NCI NIH HHS/United States
- P30 CA036727/CA/NCI NIH HHS/United States
- P30 GM103335/GM/NIGMS NIH HHS/United States
- R01 CA270234/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

