Serotonin as a biomarker of toxin-induced Parkinsonism
- PMID: 38429661
- PMCID: PMC10908133
- DOI: 10.1186/s10020-023-00773-9
Serotonin as a biomarker of toxin-induced Parkinsonism
Abstract
Background: Loss of dopaminergic neurons underlies the motor symptoms of Parkinson's disease (PD). However stereotypical PD symptoms only manifest after approximately 80% of dopamine neurons have died making dopamine-related motor phenotypes unreliable markers of the earlier stages of the disease. There are other non-motor symptoms, such as depression, that may present decades before motor symptoms.
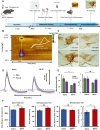
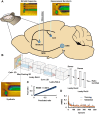
Methods: Because serotonin is implicated in depression, here we use niche, fast electrochemistry paired with mathematical modelling and machine learning to, for the first time, robustly evaluate serotonin neurochemistry in vivo in real time in a toxicological model of Parkinsonism, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
Results: Mice treated with acute MPTP had lower concentrations of in vivo, evoked and ambient serotonin in the hippocampus, consistent with the clinical comorbidity of depression with PD. These mice did not chemically respond to SSRI, as strongly as control animals did, following the clinical literature showing that antidepressant success during PD is highly variable. Following L-DOPA administration, using a novel machine learning analysis tool, we observed a dynamic shift from evoked serotonin release in the hippocampus to dopamine release. We hypothesize that this finding shows, in real time, that serotonergic neurons uptake L-DOPA and produce dopamine at the expense of serotonin, supporting the significant clinical correlation between L-DOPA and depression. Finally, we found that this post L-DOPA dopamine release was less regulated, staying in the synapse for longer. This finding is perhaps due to lack of autoreceptor control and may provide a ground from which to study L-DOPA induced dyskinesia.
Conclusions: These results validate key prior hypotheses about the roles of serotonin during PD and open an avenue to study to potentially improve therapeutics for levodopa-induced dyskinesia and depression.
Keywords: CFM; Depression; FSCV.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Abadi M, et al. TensorFlow: large-scale machine learning on heterogeneous systems. In: Proceedings of the 12th USENIX conference on Operating Systems Design and Implementation. USENIX Association. 2016. pp. 265–83. http://tensorflow.org/. Accessed 13 Aug 2016.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

