Intrafamily heterooligomerization as an emerging mechanism of methyltransferase regulation
- PMID: 38429855
- PMCID: PMC10908127
- DOI: 10.1186/s13072-024-00530-0
Intrafamily heterooligomerization as an emerging mechanism of methyltransferase regulation
Abstract
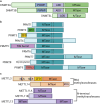
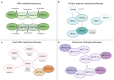
Protein and nucleic acid methylation are important biochemical modifications. In addition to their well-established roles in gene regulation, they also regulate cell signaling, metabolism, and translation. Despite this high biological relevance, little is known about the general regulation of methyltransferase function. Methyltransferases are divided into superfamilies based on structural similarities and further classified into smaller families based on sequence/domain/target similarity. While members within superfamilies differ in substrate specificity, their structurally similar active sites indicate a potential for shared modes of regulation. Growing evidence from one superfamily suggests a common regulatory mode may be through heterooligomerization with other family members. Here, we describe examples of methyltransferase regulation through intrafamily heterooligomerization and discuss how this can be exploited for therapeutic use.
Keywords: Methylation; Methyltransferase; Oligomerization; Regulation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

