Nr4a1 enhances Wnt4 transcription to promote mesenchymal stem cell osteogenesis and alleviates inflammation-inhibited bone regeneration
- PMID: 38429926
- PMCID: PMC11081873
- DOI: 10.1016/j.ymthe.2024.02.034
Nr4a1 enhances Wnt4 transcription to promote mesenchymal stem cell osteogenesis and alleviates inflammation-inhibited bone regeneration
Abstract
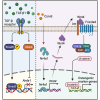
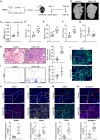
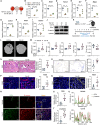
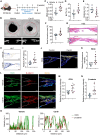
Intense inflammatory response impairs bone marrow mesenchymal stem cell (BMSC)-mediated bone regeneration, with transforming growth factor (TGF)-β1 being the most highly expressed cytokine. However, how to find effective and safe means to improve bone formation impaired by excessive TGF-β1 remains unclear. In this study, we found that the expression of orphan nuclear receptor Nr4a1, an endogenous repressor of TGF-β1, was suppressed directly by TGF-β1-induced Smad3 and indirectly by Hdac4, respectively. Importantly, Nr4a1 overexpression promoted BMSC osteogenesis and reversed TGF-β1-mediated osteogenic inhibition and pro-fibrotic effects. Transcriptomic and histologic analyses confirmed that upregulation of Nr4a1 increased the transcription of Wnt family member 4 (Wnt4) and activated Wnt pathway. Mechanistically, Nr4a1 bound to the promoter of Wnt4 and regulated its expression, thereby enhancing the osteogenic capacity of BMSCs. Moreover, treatment with Nr4a1 gene therapy or Nr4a1 agonist Csn-B could promote ectopic bone formation, defect repair, and fracture healing. Finally, we demonstrated the correlation of NR4A1 with osteogenesis and the activation of the WNT4/β-catenin pathway in human BMSCs and fracture samples. Taken together, these findings uncover the critical role of Nr4a1 in bone formation and alleviation of inflammation-induced bone regeneration disorders, and suggest that Nr4a1 has the potential to be a therapeutic target for accelerating bone healing.
Keywords: Csn-B; Nr4a1; TGF-β1; Wnt4/β-catenin; bone regeneration; mesenchymal stem cells; molecular therapy; osteogenesis.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

