Reorganization of mitochondria-organelle interactions during postnatal development in skeletal muscle
- PMID: 38429930
- PMCID: PMC10939894
- DOI: 10.1113/JP285014
Reorganization of mitochondria-organelle interactions during postnatal development in skeletal muscle
Abstract
Skeletal muscle cellular development requires the integrated assembly of mitochondria and other organelles adjacent to the sarcomere in support of muscle contractile performance. However, it remains unclear how interactions among organelles and with the sarcomere relates to the development of muscle cell function. Here, we combine 3D volume electron microscopy, proteomic analyses, and live cell functional imaging to investigate the postnatal reorganization of mitochondria-organelle interactions in skeletal muscle. We show that while mitochondrial networks are disorganized and loosely associated with the contractile apparatus at birth, contact sites among mitochondria, lipid droplets and the sarcoplasmic reticulum are highly abundant in neonatal muscles. The maturation process is characterized by a transition to highly organized mitochondrial networks wrapped tightly around the muscle sarcomere but also to less frequent interactions with both lipid droplets and the sarcoplasmic reticulum. Concomitantly, expression of proteins involved in mitochondria-organelle membrane contact sites decreases during postnatal development in tandem with a decrease in abundance of proteins associated with sarcomere assembly despite an overall increase in contractile protein abundance. Functionally, parallel measures of mitochondrial membrane potential, NADH redox status, and NADH flux within intact cells revealed that mitochondria in adult skeletal muscle fibres maintain a more activated electron transport chain compared with neonatal muscle mitochondria. These data demonstrate a developmental redesign reflecting a shift from muscle cell assembly and frequent inter-organelle communication toward a muscle fibre with mitochondrial structure, interactions, composition and function specialized to support contractile function. KEY POINTS: Mitochondrial network organization is remodelled during skeletal muscle postnatal development. The mitochondrial outer membrane is in frequent contact with other organelles at birth and transitions to more close associations with the contractile apparatus in mature muscles. Mitochondrial energy metabolism becomes more activated during postnatal development. Understanding the developmental redesign process within skeletal muscle cells may help pinpoint specific areas of deficit in muscles with developmental disorders.
Keywords: 3D mitochondrial structure; functional cellular imaging; organelle interactions; postnatal muscle development; volume electron microscopy.
Published 2024. This article is a U.S. Government work and is in the public domain in the USA. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures

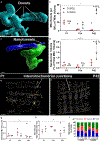

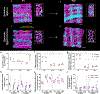



References
-
- Agbulut O, Noirez P, Beaumont F & Butler-Browne G. (2003). Myosin heavy chain isoforms in postnatal muscle development of mice. Biology of the Cell 95, 399–406. - PubMed
-
- Andrienko T, Kuznetsov AV, Kaambre T, Usson Y, Orosco A, Appaix F, Tiivel T, Sikk P, Vendelin M, Margreiter R & Saks VA. (2003). Metabolic consequences of functional complexes of mitochondria, myofibrils and sarcoplasmic reticulum in muscle cells. J Exp Biol 206, 2059–2072. - PubMed
-
- Bakeeva LE, Chentsov YS & Skulachev VP. (1981). Ontogenesis of mitochondrial reticulum in rat diaphragm muscle. Eur J Cell Biol 25, 175–181. - PubMed
-
- Bakeeva LE, Chentsov Yu S & Skulachev VP. (1978). Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim Biophys Acta 501, 349–369. - PubMed
-
- Beier T, Pape C, Rahaman N, Prange T, Berg S, Bock DD, Cardona A, Knott GW, Plaza SM, Scheffer LK, Koethe U, Kreshuk A & Hamprecht FA. (2017). Multicut brings automated neurite segmentation closer to human performance. Nat Methods 14, 101–102. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

