Cardiac-Specific Deletion of Scn8a Mitigates Dravet Syndrome-Associated Sudden Death in Adults
- PMID: 38430092
- PMCID: PMC11285447
- DOI: 10.1016/j.jacep.2024.01.003
Cardiac-Specific Deletion of Scn8a Mitigates Dravet Syndrome-Associated Sudden Death in Adults
Abstract
Background: Sudden unexpected death in epilepsy (SUDEP) is a fatal complication experienced by otherwise healthy epilepsy patients. Dravet syndrome (DS) is an inherited epileptic disorder resulting from loss of function of the voltage-gated sodium channel, NaV 1.1, and is associated with particularly high SUDEP risk. Evidence is mounting that NaVs abundant in the brain also occur in the heart, suggesting that the very molecular mechanisms underlying epilepsy could also precipitate cardiac arrhythmias and sudden death. Despite marked reduction of NaV 1.1 functional expression in DS, pathogenic late sodium current (INa,L) is paradoxically increased in DS hearts. However, the mechanisms by which DS directly impacts the heart to promote sudden death remain unclear.
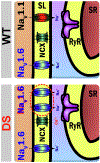
Objectives: In this study, the authors sought to provide evidence implicating remodeling of Na+ - and Ca2+ -handling machinery, including NaV 1.6 and Na+/Ca2+exchanger (NCX) within transverse (T)-tubules in DS-associated arrhythmias.
Methods: The authors undertook scanning ion conductance microscopy (SICM)-guided patch clamp, super-resolution microscopy, confocal Ca2+ imaging, and in vivo electrocardiography studies in Scn1a haploinsufficient murine model of DS.
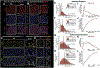
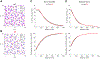
Results: DS promotes INa,L in T-tubular nanodomains, but not in other subcellular regions. Consistent with increased NaV activity in these regions, super-resolution microscopy revealed increased NaV 1.6 density near Ca2+release channels, the ryanodine receptors (RyR2) and NCX in DS relative to WT hearts. The resulting INa,L in these regions promoted aberrant Ca2+ release, leading to ventricular arrhythmias in vivo. Cardiac-specific deletion of NaV 1.6 protects adult DS mice from increased T-tubular late NaV activity and the resulting arrhythmias, as well as sudden death.
Conclusions: These data demonstrate that NaV 1.6 undergoes remodeling within T-tubules of adult DS hearts serving as a substrate for Ca2+ -mediated cardiac arrhythmias and may be a druggable target for the prevention of SUDEP in adult DS subjects.
Keywords: Dravet syndrome; Na(V)1.6; sodium channels; sudden cardiac death; sudden unexpected death in epilepsy.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This work was supported by National Institutes of Health grants R01NS121234 and R01HL155378 (to Dr Radwański), R01HL148736 (to Dr Veeraraghavan), R01HL156652 (to Dr Hund), K99HL157684 (to Dr Nassal), and T32HL149637-02 and L40NS129034 (to Dr King); National Science Foundation Graduate Research Fellowship, NSF Fellow ID: 2019259354 (to Dr Struckman); and American Heart Association postdoctoral fellowships 915300 (to Dr Tarasov) and 908824 (to Dr Moise). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures




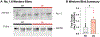


References
-
- Skluzacek JV, Watts KP, Parsy O, Wical B, Camfield P. Dravet syndrome and parent associations: the IDEA League experience with comorbid conditions, mortality, management, adaptation, and grief. Epilepsia. 2011;52(suppl 2):95–101. - PubMed
-
- Delogu AB, Spinelli A, Battaglia D, et al. Electrical and autonomic cardiac function in patients with Dravet syndrome: cardiac autonomic function in Dravet syndrome. Epilepsia. 2011;52:55–58. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

