ITF2357 regulates NF-κB signaling pathway to protect barrier integrity in retinal pigment epithelial cells
- PMID: 38430220
- PMCID: PMC11019659
- DOI: 10.1096/fj.202301592R
ITF2357 regulates NF-κB signaling pathway to protect barrier integrity in retinal pigment epithelial cells
Abstract
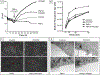
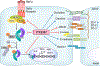
The robust integrity of the retinal pigment epithelium (RPE), which contributes to the outer brain retina barrier (oBRB), is compromised in several retinal degenerative and vascular disorders, including diabetic macular edema (DME). This study evaluates the role of a new generation of histone deacetylase inhibitor (HDACi), ITF2357, in regulating outer blood-retinal barrier function and investigates the underlying mechanism of action in inhibiting TNFα-induced damage to RPE integrity. Using the immortalized RPE cell line (ARPE-19), ITF2357 was found to be non-toxic between 50 nM and 5 μM concentrations. When applied as a pre-treatment in conjunction with an inflammatory cytokine, TNFα, the HDACi was safe and effective in preventing epithelial permeability by fortifying tight junction (ZO-1, -2, -3, occludin, claudin-1, -2, -3, -5, -19) and adherens junction (E-cadherin, Nectin-1) protein expression post-TNFα stress. Mechanistically, ITF2357 depicted a late action at 24 h via attenuating IKK, IκBα, and p65 phosphorylation and ameliorated the expression of IL-1β, IL-6, and MCP-1. Also, ITF2357 delayed IκBα synthesis and turnover. The use of Bay 11-7082 and MG132 further uncovered a possible role for ITF2357 in non-canonical NF-κB activation. Overall, this study revealed the protection effects of ITF2357 by regulating the turnover of tight and adherens junction proteins and modulating NF-κB signaling pathway in the presence of an inflammatory stressor, making it a potential therapeutic application for retinal vascular diseases such as DME with compromised outer blood-retinal barrier.
Keywords: HDAC inhibitor; ITF2357; NF-κB; diabetic macular edema; inflammation; outer blood-retinal barrier; retinal pigment epithelium.
© 2024 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures





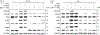
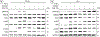
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

