Mechanism exploration of Zoledronic acid combined with PD-1 in the treatment of hepatocellular carcinoma
- PMID: 38430249
- PMCID: PMC10908605
- DOI: 10.1007/s00262-024-03652-2
Mechanism exploration of Zoledronic acid combined with PD-1 in the treatment of hepatocellular carcinoma
Abstract
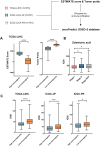
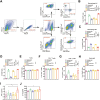
How to increase the response of immune checkpoint inhibitors (ICIs) is a challenge. In clinical, we found that Zoledronic acid (ZA) may increase the anti-tumor effect of immunotherapy for hepatocellular carcinoma (HCC). To explore the underlying mechanism, we established a mouse model of HCC by subcutaneously injecting Hepa1-6 cell line. The result showed that the tumor volume in the ZA plus anti-PD-1 monocloning antibody (anti-PD-1 mAb) treatment groups was significantly smaller than that of control group, and the onset time of tumor inhibition was even shorter than that of the anti-PD-1 mAb group. Using flow cytometry (FC) to detect the proportion of major immune cell subsets in tumor tissues of each group of mice, we found that the synergistic anti-tumor effect of ZA and anti-PD-1 mAb may be related to ZA-induced polarization of macrophages toward the M1 phenotype. Next, we performed bulk RNA sequencing on tumor samples from different groups to obtain differentially expressed genes (DEGs), which were then input DEGs into pathway enrichment analysis. Data indicated that ZA participated in the M1-type polarization via ferroptosis-related pathways. Our results revealed how ZA involves in the anti-tumor effect of PD-1 monoclonal antibody and provided a potential therapeutic candidate for patients with HCC.
Keywords: Hepatocellular carcinoma; Macrophage; PD-1 antibody; Zoledronic acid.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Inhibition of APOC1 promotes the transformation of M2 into M1 macrophages via the ferroptosis pathway and enhances anti-PD1 immunotherapy in hepatocellular carcinoma based on single-cell RNA sequencing.Redox Biol. 2022 Oct;56:102463. doi: 10.1016/j.redox.2022.102463. Epub 2022 Sep 2. Redox Biol. 2022. PMID: 36108528 Free PMC article.
-
Bufalin stimulates antitumor immune response by driving tumor-infiltrating macrophage toward M1 phenotype in hepatocellular carcinoma.J Immunother Cancer. 2022 May;10(5):e004297. doi: 10.1136/jitc-2021-004297. J Immunother Cancer. 2022. PMID: 35618286 Free PMC article.
-
Zoledronic acid inhibits thyroid cancer stemness and metastasis by repressing M2-like tumor-associated macrophages induced Wnt/β-catenin pathway.Life Sci. 2020 Sep 1;256:117925. doi: 10.1016/j.lfs.2020.117925. Epub 2020 Jun 6. Life Sci. 2020. PMID: 32522570
-
Novel antigens for targeted radioimmunotherapy in hepatocellular carcinoma.Mol Cell Biochem. 2023 Jan;478(1):23-37. doi: 10.1007/s11010-022-04483-4. Epub 2022 Jun 16. Mol Cell Biochem. 2023. PMID: 35708866 Review.
-
Viral status, immune microenvironment and immunological response to checkpoint inhibitors in hepatocellular carcinoma.J Immunother Cancer. 2020 Apr;8(1):e000394. doi: 10.1136/jitc-2019-000394. J Immunother Cancer. 2020. PMID: 32303615 Free PMC article.
Cited by
-
Fucoidan-decorated metal-zoledronic acid nanocomplexes suppress tumor metastasis by inducing ferroptotic cell death and enhancing cancer immunotherapy.J Nanobiotechnology. 2025 Jun 2;23(1):405. doi: 10.1186/s12951-025-03473-0. J Nanobiotechnology. 2025. PMID: 40452005 Free PMC article.
-
Unveiling the Therapeutic Potential of Trigonelline: A Promising Approach in Cancer Prevention and Treatment.Anticancer Agents Med Chem. 2025;25(16):1175-1187. doi: 10.2174/0118715206363456250226061713. Anticancer Agents Med Chem. 2025. PMID: 40045859 Review.
-
Interactions between tumor-associated macrophages and regulated cell death: therapeutic implications in immuno-oncology.Front Oncol. 2024 Nov 7;14:1449696. doi: 10.3389/fonc.2024.1449696. eCollection 2024. Front Oncol. 2024. PMID: 39575419 Free PMC article. Review.
References
-
- Yang W, Soares J, Greninger P, Edelman EJ, Lightfoot H, Forbes S, Bindal N, Beare D, Smith JA, Thompson IR, et al. Genomics of drug sensitivity in cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2012;41(D1):D955–D961. doi: 10.1093/nar/gks1111. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

