Early intensive versus escalation treatment in patients with relapsing-remitting multiple sclerosis in Austria
- PMID: 38430270
- PMCID: PMC11136709
- DOI: 10.1007/s00415-024-12256-w
Early intensive versus escalation treatment in patients with relapsing-remitting multiple sclerosis in Austria
Abstract
Objectives: To compare the effectiveness of early intensive treatment (EIT) versus escalation treatment (ESC) in a nationwide observational cohort of almost 1000 people with relapsing-remitting multiple sclerosis (RRMS).
Materials and methods: The EIT cohort started with alemtuzumab (AZM), cladribine (CLAD), fingolimod (FTY), natalizumab (NTZ), ocrelizumab (OCR), or ozanimod (OZA); whereas, the ESC cohort was escalated from dimethylfumarate (DMF) or teriflunomide (TERI) to AZM, CLAD, FTY, NTZ, OCR, or OZA within the Austrian MS Treatment Registry. Patients had to stay on therapy for at least 3 months and up to 16 years. The EIT cohort included 743 and the ESC cohort 227 RRMS patients. We used multinomial propensity scores for inverse probability weighting in generalized linear (GLM) and Cox proportional hazards models to correct for the bias of this non-randomized registry study.
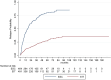
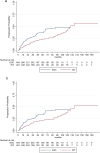
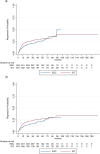
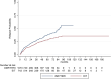
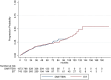
Results: Estimated mean annualized relapse rates (ARR) were 0.09 for EIT and 0.4 for ESC patients. The incidence rate ratio (IRR) in the GLM model for relapses showed a decreased relapse probability of 78% for the EIT versus ESC cohort [IRR = 0.22, 95% CI (0.16-0.30), p < 0.001]. Analyzing the time to the first relapse by Cox regression, a hazard ratio (HR) of 0.17 [95% CI (0.13-0.22), p < 0.001] revealed a decreased risk of 83% for the EIT group. Regarding sustained Expanded Disability Status Scale (EDSS) progression for 12 weeks, a HR of 0.55 [95% CI (0.40-0.76), p < 0.001] showed a decreased probability of 45% for the EIT cohort.
Conclusions: ESC treatment after DMF and TERI revealed a higher relapse and EDSS progression probability compared to EIT in Austrian RRMS patients. Therefore, an early intensive treatment should be started in patients with an active or highly active disease course.
Keywords: Comparison; Early intensive treatment; Escalation treatment; Multiple sclerosis.
© 2024. The Author(s).
Conflict of interest statement
Michael Guger received support and honoraria for research, consultation, lectures and education from Alexion, Almirall, Bayer, Biogen, Bristol-Myers-Squibb, Celgene, Genzyme, Horizon, Janssen-Cilag, MedDay, Merck, Novartis, Roche, Sanofi Aventis and TEVA ratiopharm. Christian Enzinger received funding for travel and speaker honoraria from Bayer, Biogen, Genzyme, Merck, Novartis, Roche, Shire, and Teva Pharmaceutical Industries Ltd./sanofi-aventis, research support from Biogen, Merck, and Teva Pharmaceutical Industries Ltd./sanofi-aventis and serving on scientific advisory boards for Bayer, Biogen, Merck, Novartis, Roche and Teva Pharmaceutical Industries Ltd./sanofi- Aventis. Fritz Leutmezer has received funding for travel and speaker honoraria from Actelion, Almirall, Bayer, Biogen, Bristol-Myers-Squibb, Celgene, Genzyme, Janssen-Cilag, MedDay, Merck, Novartis, Pfizer, Octapharm, Roche, Sanofi-Genzyme and Teva. Franziska Di Pauli has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations) or travel funding from Bayer, Biogen, Celgene, Merck, Novartis, Sanofi-Genzyme, Roche and Teva. Jörg Kraus received consulting and/or research funding and/or educational support from Alexion, Almirall, Bayer, Biogen, Bristol-Myers-Squibb, Horizon, Janssen-Cilag, MedDay, Medtronic, Merck, Novartis, Roche, Sandoz, Sanofi-Aventis, Shire, TEVA ratiopharm. Stefan Kalcher declares that there is no conflict of interest. Erich Kvas declares that there is no conflict of interest. Thomas Berger has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for multiple sclerosis: Almirall, Bayer, Biogen, Biologix, Bionorica, Bristol-Myers-Squibb, Eisai, GW Pharma, Horizon, Janssen-Cilag, MedDay, Merck, Novartis, Octapharma, Roche, Sandoz, Sanofi/Genzyme, TG Pharmaceuticals, TEVA-ratiopharm and UCB. His institution has received financial support in the last 12 months by unrestricted research grants (Biogen, Bayer, Bristol-Myers-Squibb, Merck, Novartis, Sanofi/Genzyme, and TEVA ratiopharm) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Bristol-Myers-Squibb, Merck, Novartis, Octapharma, Roche, Sanofi/Genzyme, and TEVA.
Figures
References
-
- Brown JWL, Coles A, Horakova D, Havrdova E, Izquierdo G, Prat A, Girard M, Duquette P, Trojano M, Lugaresi A, Bergamaschi R, Grammond P, Alroughani R, Hupperts R, McCombe P, Van Pesch V, Sola P, Ferraro D, Grand’Maison F, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA. 2019;321(2):175. doi: 10.1001/jama.2018.20588. - DOI - PMC - PubMed
-
- Calabresi PA, Radue E-W, Goodin D, Jeffery D, Rammohan KW, Reder AT, Vollmer T, Agius MA, Kappos L, Stites T, Li B, Cappiello L, von Rosenstiel P, Lublin FD. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545–556. doi: 10.1016/S1474-4422(14)70049-3. - DOI - PubMed
-
- Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung H-P, Havrdova E, Selmaj KW, Weiner HL, Fisher E, Brinar VV, Giovannoni G, Stojanovic M, Ertik BI, Lake SL, Margolin DH, Panzara MA, Compston DAS, CARE-MS I investigators Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet (London, England) 2012;380(9856):1819–1828. doi: 10.1016/S0140-6736(12)61769-3. - DOI - PubMed
-
- Cohen JA, Comi G, Selmaj KW, Bar-Or A, Arnold DL, Steinman L, Hartung H-P, Montalban X, Kubala Havrdová E, Cree BAC, Sheffield JK, Minton N, Raghupathi K, Huang V, Kappos L, Trial RADIANCE, Investigators. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18(11):1021–1033. doi: 10.1016/S1474-4422(19)30238-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

