A Nanographene-Porphyrin Hybrid for Near-Infrared-Ii Phototheranostics
- PMID: 38430537
- PMCID: PMC11095198
- DOI: 10.1002/advs.202309131
A Nanographene-Porphyrin Hybrid for Near-Infrared-Ii Phototheranostics
Abstract
Photoacoustic imaging (PAI)-guided photothermal therapy (PTT) in the second near-infrared (NIR-II, 1000-1700 nm) window has been attracting attention as a promising cancer theranostic platform. Here, it is reported that the π-extended porphyrins fused with one or two nanographene units (NGP-1 and NGP-2) can serve as a new class of NIR-responsive organic agents, displaying absorption extending to ≈1000 and ≈1400 nm in the NIR-I and NIR-II windows, respectively. NGP-1 and NGP-2 are dispersed in water through encapsulation into self-assembled nanoparticles (NPs), achieving high photothermal conversion efficiency of 60% and 69%, respectively, under 808 and 1064 nm laser irradiation. Moreover, the NIR-II-active NGP-2-NPs demonstrated promising photoacoustic responses, along with high photostability and biocompatibility, enabling PAI and efficient NIR-II PTT of cancer in vivo.
Keywords: NIR‐II absorption; nanographene; photoacoustic imaging; photothermal therapy; porphyrin.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


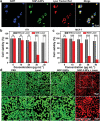
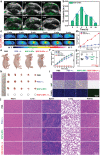
Similar articles
-
Ultrathin Two-Dimensional Plasmonic PtAg Nanosheets for Broadband Phototheranostics in Both NIR-I and NIR-II Biowindows.Adv Sci (Weinh). 2021 Sep;8(17):e2100386. doi: 10.1002/advs.202100386. Epub 2021 Jul 11. Adv Sci (Weinh). 2021. PMID: 34247445 Free PMC article.
-
Small-Molecule Phototheranostic Agent with Extended π-Conjugation for Efficient NIR-II Photoacoustic-Imaging-Guided Photothermal Therapy.Small. 2024 Apr;20(17):e2307829. doi: 10.1002/smll.202307829. Epub 2023 Dec 3. Small. 2024. PMID: 38044585
-
De novo strategy of organic semiconducting polymer brushes for NIR-II light-triggered carbon monoxide release to boost deep-tissue cancer phototheranostics.J Nanobiotechnology. 2024 Nov 14;22(1):708. doi: 10.1186/s12951-024-02984-6. J Nanobiotechnology. 2024. PMID: 39543646 Free PMC article.
-
Second Near-Infrared Plasmonic Nanomaterials for Photoacoustic Imaging and Photothermal Therapy.Small. 2023 Jul;19(30):e2300539. doi: 10.1002/smll.202300539. Epub 2023 Apr 14. Small. 2023. PMID: 37060228 Review.
-
Applications of nanotheranostics in the second near-infrared window in bioimaging and cancer treatment.Nanoscale. 2024 Dec 5;16(47):21697-21730. doi: 10.1039/d4nr03058c. Nanoscale. 2024. PMID: 39508492 Review.
Cited by
-
Donor modulation brings all-in-one phototheranostics for NIR-II imaging-guided type-I photodynamic/photothermal synergistic cancer therapy.Chem Sci. 2025 Feb 4;16(12):5089-5098. doi: 10.1039/d4sc08685f. eCollection 2025 Mar 19. Chem Sci. 2025. PMID: 39968281 Free PMC article.
-
Manifold-Fused Porphyrin-Nanographene Conjugates.Chemistry. 2024 Dec 10;30(69):e202403250. doi: 10.1002/chem.202403250. Epub 2024 Nov 16. Chemistry. 2024. PMID: 39410809 Free PMC article.
-
Panchromatic PAH-Porphyrin Hybrids with a Step-Wise Increasing π-System.ChemistryOpen. 2025 Mar;14(3):e202400481. doi: 10.1002/open.202400481. Epub 2025 Feb 11. ChemistryOpen. 2025. PMID: 39935043 Free PMC article.
-
A single-molecule graphene quantum dot: a novel efficient photosensitizer for photodynamic cancer therapy.Chem Sci. 2025 Jun 30;16(30):13923-13934. doi: 10.1039/d5sc03226a. eCollection 2025 Jul 30. Chem Sci. 2025. PMID: 40630627 Free PMC article.
-
Recent advances of photoresponsive nanomaterials for diagnosis and treatment of acute kidney injury.J Nanobiotechnology. 2024 Nov 5;22(1):676. doi: 10.1186/s12951-024-02906-6. J Nanobiotechnology. 2024. PMID: 39501286 Free PMC article. Review.
References
-
- Ng K. K., Zheng G., Chem. Rev. 2015, 115, 11012. - PubMed
-
- Cheng L., Wang C., Feng L. Z., Yang K., Liu Z., Chem. Rev. 2014, 114, 10869. - PubMed
-
- Yan D. Y., Wang M., Wu Q., Niu N., Li M., Song R. X., Rao J., Kang M. M., Zhang Z. J., Zhou F. F., Wang D., Tang B. Z., Angew. Chem., Int. Ed. 2022, 61, e2022026. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
