Remodeling of the Intra-Conduit Inflammatory Microenvironment to Improve Peripheral Nerve Regeneration with a Neuromechanical Matching Protein-Based Conduit
- PMID: 38430538
- PMCID: PMC11077661
- DOI: 10.1002/advs.202302988
Remodeling of the Intra-Conduit Inflammatory Microenvironment to Improve Peripheral Nerve Regeneration with a Neuromechanical Matching Protein-Based Conduit
Abstract
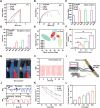
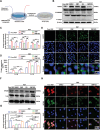
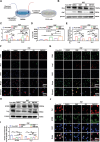
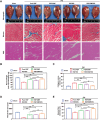
Peripheral nerve injury (PNI) remains a challenging area in regenerative medicine. Nerve guide conduit (NGC) transplantation is a common treatment for PNI, but the prognosis of NGC treatment is unsatisfactory due to 1) neuromechanical unmatching and 2) the intra-conduit inflammatory microenvironment (IME) resulting from Schwann cell pyroptosis and inflammatory-polarized macrophages. A neuromechanically matched NGC composed of regenerated silk fibroin (RSF) loaded with poly(3,4-ethylenedioxythiophene): poly(styrene sulfonate) (P:P) and dimethyl fumarate (DMF) are designed, which exhibits a matched elastic modulus (25.1 ± 3.5 MPa) for the peripheral nerve and the highest 80% elongation at break, better than most protein-based conduits. Moreover, the NGC can gradually regulate the intra-conduit IME by releasing DMF and monitoring sciatic nerve movements via piezoresistive sensing. The combination of NGC and electrical stimulation modulates the IME to support PNI regeneration by synergistically inhibiting Schwann cell pyroptosis and reducing inflammatory factor release, shifting macrophage polarization from the inflammatory M1 phenotype to the tissue regenerative M2 phenotype and resulting in functional recovery of neurons. In a rat sciatic nerve crush model, NGC promoted remyelination and functional and structural regeneration. Generally, the DMF/RSF/P:P conduit provides a new potential therapeutic approach to promote nerve repair in future clinical treatments.
Keywords: electrical stimulation; intra‐conduit inflammatory microenvironment; neuromechanical matching; peripheral nerve regeneration; silk fibroin.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Jin X. H., Fang J. Q., Wang J. G., Xu B., Wang X., Liu S. H., Chen F., Liu J. J., J. Mater. Chem. B. 2022, 10, 8771. - PubMed
-
- Zhang G., Ma L., Bai L., Li M., Guo T., Tian B., He Z., Fu Q., J. Control Rel. 2021, 334, 114. - PubMed
-
- Li W., Liang J., Li S., Wang L., Xu S., Jiang S., Song M., Meng H., Zhai D., Tang L., Yang Y., Zhang L., Zhang B., Int. Immunopharmacol. 2022, 110, 109026. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82272457/National Natural Science Foundation of China
- 2020YFC2008400/National Key Research and Development Program of China
- 2023CXGC010509/Key Research & Developmental Program of Shandong Province
- 202328050/Science and Technology Plan Project of Jinan
- 21S11902700/Technology Innovation Action Plan" of Shanghai Science and Technology Commission
LinkOut - more resources
Full Text Sources
Medical
