An autologous ex vivo model for exploring patient-specific responses to viro-immunotherapy in glioblastoma
- PMID: 38430913
- PMCID: PMC10985229
- DOI: 10.1016/j.crmeth.2024.100716
An autologous ex vivo model for exploring patient-specific responses to viro-immunotherapy in glioblastoma
Abstract
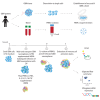
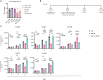
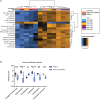
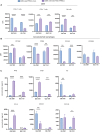
Oncolytic virus (OV) clinical trials have demonstrated remarkable efficacy in subsets of patients with glioblastoma (GBM). However, the lack of tools to predict this response hinders the advancement of a more personalized application of OV therapy. In this study, we characterize an ex vivo co-culture system designed to examine the immune response to OV infection of patient-derived GBM neurospheres in the presence of autologous peripheral blood mononuclear cells (PBMCs). Co-culture conditions were optimized to retain viability and functionality of both tumor cells and PBMCs, effectively recapitulating the well-recognized immunosuppressive effects of GBM. Following OV infection, we observed elevated secretion of pro-inflammatory cytokines and chemokines, including interferon γ, tumor necrosis factor α, CXCL9, and CXCL10, and marked changes in immune cell activation markers. Importantly, OV treatment induced unique patient-specific immune responses. In summary, our co-culture platform presents an avenue for personalized screening of viro-immunotherapies in GBM, offering promise as a potential tool for future patient stratification in OV therapy.
Keywords: CP: Cancer biology; Delta24-RGD; GBM neurospheres; PBMCs; autologous co-culture; co-culture model; glioblastoma; immune response; individualized screening; oncolytic viruses; spectral flow cytometry.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


References
-
- De Witt Hamer P.C., Ho V.K.Y., Zwinderman A.H., Ackermans L., Ardon H., Boomstra S., Bouwknegt W., van den Brink W.A., Dirven C.M., van der Gaag N.A., et al. Between-hospital variation in mortality and survival after glioblastoma surgery in the Dutch Quality Registry for Neuro Surgery. J. Neuro Oncol. 2019;144:313–323. - PMC - PubMed
-
- Lang F.F., Conrad C., Gomez-Manzano C., Yung W.K.A., Sawaya R., Weinberg J.S., Prabhu S.S., Rao G., Fuller G.N., Aldape K.D., et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018;36:1419–1427. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

