Smart exosomes enhance PDAC targeted therapy
- PMID: 38431093
- PMCID: PMC12312550
- DOI: 10.1016/j.jconrel.2024.02.037
Smart exosomes enhance PDAC targeted therapy
Abstract
Exosomes continue to attract interest as a promising nanocarrier drug delivery technology. They are naturally derived nanoscale extracellular vesicles with innate properties well suited to shuttle proteins, lipids, and nucleic acids between cells. Nonetheless, their clinical utility is currently limited by several major challenges, such as their inability to target tumor cells and a high proportion of clearance by the mononuclear phagocyte system (MPS) of the liver and spleen. To overcome these limitations, we developed "Smart Exosomes" that co-display RGD and CD47p110-130 through CD9 engineering (ExoSmart). The resultant ExoSmart demonstrates enhanced binding capacity to αvβ3 on pancreatic ductal adenocarcinoma (PDAC) cells, resulting in amplified cellular uptake in in vitro and in vivo models and increased chemotherapeutic efficacies. Simultaneously, ExoSmart significantly reduced liver and spleen clearance of exosomes by inhibiting macrophage phagocytosis via CD47p110-130 interaction with signal regulatory proteins (SIRPα) on macrophages. These studies demonstrate that an engineered exosome drug delivery system increases PDAC therapeutic efficacy by enhancing active PDAC targeting and prolonging circulation times, and their findings hold tremendous translational potential for cancer therapy while providing a concrete foundation for future work utilizing novel peptide-engineered exosome strategies.
Keywords: Bioengineering; CD47; CD9; Drug delivery; Exosomes; Gemcitabine; Paclitaxel; Pancreatic cancer; RGD.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that there is no conflict of interest in this study.
Figures



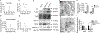




References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A, Cancer Statistics, 2021, CA Cancer J Clin, 71 (2021) 7–33. - PubMed
-
- Krupa G, Jani RK, Active targeting of nanoparticles: An innovative technology for drug delivery in cancer therapeutics, Journal of Drug Delivery and Therapeutics, 9 (2019) 408–415.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

