Safety and immunogenicity of Innovax bivalent human papillomavirus vaccine in girls 9-14 years of age: Interim analysis from a phase 3 clinical trial
- PMID: 38431444
- PMCID: PMC11007388
- DOI: 10.1016/j.vaccine.2024.02.077
Safety and immunogenicity of Innovax bivalent human papillomavirus vaccine in girls 9-14 years of age: Interim analysis from a phase 3 clinical trial
Abstract
Background: World Health Organization human papillomavirus (HPV) vaccination recommendations include a single- or two-dose schedule in individuals 9-20 years old and advice for generating data on single-dose efficacy or immunobridging. The ongoing Phase 3 trial of Innovax's bivalent (types 16 and 18) HPV vaccine (Cecolin®) assesses in low- and middle-income countries alternative dosing schedules and generates data following one dose in girls 9-14 years old. Interim data for the 6-month dosing groups are presented.
Methods: In Bangladesh and Ghana, 1,025 girls were randomized to receive either two doses of Cecolin at 6-, 12-, or 24-month intervals; one dose of Gardasil® followed by one dose of Cecolin at month 24; or two doses of Gardasil 6 months apart (referent). Serology was measured by enzyme-linked immunosorbent assay (ELISA) and, in a subset, by neutralization assays. Primary objectives include immunological non-inferiority of the Cecolin schedules to referent one month after the second dose. Safety endpoints include reactogenicity and unsolicited adverse events for 7 and 30 days post-vaccination, respectively, as well as serious adverse events throughout the study.
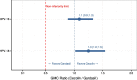
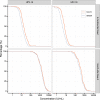
Results: Interim analyses included data from the two groups on a 0, 6-month schedule with 205 participants per group. One month after Dose 2, 100% of participants were seropositive by ELISA and had seroconverted for both antigens. Non-inferiority of Cecolin to Gardasil was demonstrated. Six months following one dose, over 96% of participants were seropositive by ELISA for both HPV antigens, with a trend for higher geometric mean concentration following Cecolin administration. Reactogenicity and safety were comparable between both vaccines.
Conclusions: Cecolin in a 0, 6-month schedule elicits robust immunogenicity. Non-inferiority to Gardasil was demonstrated one month after a 0, 6-month schedule. Immunogenicity following one dose was comparable to Gardasil up to six months. Both vaccines were safe and well tolerated (ClinicalTrials.gov No. 04508309).
Trial registration: ClinicalTrials.gov NCT04508309.
Keywords: HPV vaccine; Human papillomavirus (HPV); Immunization; Immunobridging; One-dose immunogenicity.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Financial support for this study was provided by the Bill & Melinda Gates Foundation in Seattle, Washington, United States, and the Federal Ministry of Education and Research in Frankfurt, Germany. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation. Financial support to icddr,b is provided by the governments of Bangladesh and Canada. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. 75N91019D00024. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government. Aside from the funding provided for this study as described above, the authors do not have any other conflict of interest to declare.
Figures

References
-
- National Cancer Institute. HPV and Cancer page, https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-ag...; 2019 [accessed July 12, 2023].
-
- International Agency for Research on Cancer, Global Cancer Observatory: Cancer Today page, http://gco.iarc.fr/today; 2020 [accessed June 9, 2023.].
-
- Bruni L, Albero G, Serrano B, Mena M, Collado JJ, Gómez D, et al. Human Papillomavirus and Related Diseases Report. Barcelona, Spain: ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre); 2023. https://hpvcentre.net/statistics/reports/XWX.pdf.
-
- Gu Y, Wei M, Wang D, Li Z, Xie M, Pan H, et al. Characterization of an Escherichia coli-derived human papillomavirus type 16 and 18 bivalent vaccine. Vaccine. 2017;35(35 Pt B):4637–4645. doi:10.1016/j.vaccine.2017.06.084. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

