Th17/1 and ex-Th17 cells are detected in patients with polyarticular juvenile arthritis and increase following treatment
- PMID: 38431635
- PMCID: PMC10908086
- DOI: 10.1186/s12969-024-00965-5
Th17/1 and ex-Th17 cells are detected in patients with polyarticular juvenile arthritis and increase following treatment
Abstract
Background: A better understanding of the pathogenesis of polyarticular juvenile idiopathic arthritis (polyJIA) is needed to aide in the development of data-driven approaches to guide selection between therapeutic options. One inflammatory pathway of interest is JAK-STAT signaling. STAT3 is a transcription factor critical to the differentiation of inflammatory T helper 17 cells (Th17s). Previous studies have demonstrated increased STAT3 activation in adult patients with rheumatoid arthritis, but less is known about STAT3 activation in polyJIA. We hypothesized that Th17 cells and STAT3 activation would be increased in treatment-naïve polyJIA patients compared to pediatric controls.
Methods: Blood from 17 patients with polyJIA was collected at initial diagnosis and again if remission was achieved (post-treatment). Pediatric healthy controls were also collected. Peripheral blood mononuclear cells were isolated and CD4 + T cell subsets and STAT activation (phosphorylation) were evaluated using flow cytometry. Data were analyzed using Mann-Whitney U and Wilcoxon matched-pairs signed rank tests.
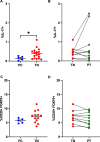
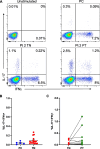
Results: Treatment-naïve polyJIA patients had increased Th17 cells (CD3 + CD4 + interleukin(IL)-17 +) compared to controls (0.15% v 0.44%, p < 0.05), but Tregs (CD3 + CD4 + CD25 + FOXP3 +) from patients did not differ from controls. Changes in STAT3 phosphorylation in CD4 + T cells following ex vivo stimulation were not significantly different in patients compared to controls. We identified dual IL-17 + and interferon (IFN)γ + expressing CD4 + T cells in patients, but not controls. Further, both Th17/1 s (CCR6 + CD161 + IFNγ + IL-17 +) and ex-Th17s (CCR6 + CD161 + IFNγ + IL-17neg) were increased in patients' post-treatment (Th17/1: 0.3% v 0.07%, p < 0.05 and ex-Th17s: 2.3% v 1.4%, p < 0.05). The patients with the highest IL-17 expressing cells post-treatment remained therapy-bound.
Conclusions: Patients with polyJIA have increased baseline Th17 cells, potentially reflecting higher tonic STAT3 activation in vivo. These quantifiable immune markers may identify patients that would benefit upfront from pathway-focused biologic therapies. Our data also suggest that inflammatory CD4 + T cell subsets not detected in controls but increased in post-treatment samples should be further evaluated as a tool to stratify patients in remission on medication. Future work will explore these proposed diagnostic and prognostic biomarkers.
Keywords: Ex-Th17; IL-6; Polyarticular juvenile idiopathic arthritis; STAT3; Th17; Th17/1; Treg.
© 2024. The Author(s).
Conflict of interest statement
TPV has consulted for Novartis, Pfizer, Moderna and SOBI and receives research support from AstraZeneca. All other authors report no disclosures.
Figures


References
-
- Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. The Lancet. 2011;377(9783):2138–2149. - PubMed
-
- Ravelli A, Consolaro A, Horneff G, Laxer RM, Lovell DJ, Wulffraat NM, et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2018;77:819. - PubMed
-
- Mannion ML, Cron RQ. Therapeutic strategies for treating juvenile idiopathic arthritis. Curr Opin Pharmacol. 2022;64:102226. - PubMed
-
- Ringold S, Angeles Han ST, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for non systemic Polyarthritis, Sacroiliitis, and Enthesitis. Arthritis Care Res. 2019;71(6):717–734. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

