Cobalt-Catalyzed Enantioselective Hydroboration of α-Substituted Acrylates
- PMID: 38431968
- PMCID: PMC11407689
- DOI: 10.1021/jacs.3c12020
Cobalt-Catalyzed Enantioselective Hydroboration of α-Substituted Acrylates
Abstract
Even though metal-catalyzed enantioselective hydroborations of alkenes have attracted enormous attention, few preparatively useful reactions of α-alkyl acrylic acid derivatives are known, and most use rhodium catalysts. No examples of asymmetric hydroboration of the corresponding α-arylacrylic acid esters are known. In our continuing efforts to search for new applications of earth-abundant cobalt catalysts for broadly applicable organic transformations, we have identified 2-(2-diarylphosphinophenyl)oxazoline ligands and mild reaction conditions for efficient and highly regio- and enantioselective hydroboration of α-alkyl- and α-aryl- acrylates, giving β-borylated propionates. Since the C-B bonds in these compounds can be readily replaced by C-O, C-N, and C-C bonds, these intermediates could serve as valuable chiral synthons, some from feedstock carbon sources, for the synthesis of propionate-bearing motifs including polyketides and related molecules. Two-step syntheses of "Roche" ester from methyl methacrylate (79%; er 99:1), arguably the most widely used chiral fragment in polyketide synthesis, and tropic acid esters (∼80% yield; er ∼93:7), which are potential intermediates for several medicinally important classes of compounds, illustrate the power of the new methods. Mechanistic studies confirm the requirement of a cationic Co(I) species [(L)Co]+as the viable catalyst in these reactions and rule out the possibility of a [L]Co-H-initiated route, which has been well-established in related hydroborations of other classes of alkenes. A mechanism involving an oxidative migration of a boryl group to the β-carbon of an η4-coordinated acrylate-cobalt complex is proposed as a plausible route.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




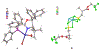





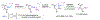


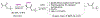



References
-
- Rentsch A; Landsberg D; Brodmann T; Bulow L; Girbig AK; Kalesse M Synthesis and pharmacology of proteasome inhibitors. Angew. Chem. Int. Ed 2013, 52, 5450–5488. - PubMed
- Smoum R; Rubinstein A; Dembitsky VM; Srebnik M Boron containing compounds as protease inhibitors. Chem. Rev 2012, 112, 4156–4220. - PubMed
- Touchet S; Carreaux F; Carboni B; Bouillon A; Boucher JL Aminoboronic acids and esters: From synthetic challenges to the discovery of unique classes of enzyme inhibitors. Chem. Soc. Rev 2011, 40, 3895–3914. - PubMed
- Dembitsky VM; Al Quntar AA; Srebnik M Natural and synthetic small boron-containing molecules as potential inhibitors of bacterial and fungal quorum sensing. Chem. Rev 2011, 111, 209–237. - PubMed
-
- Mellerup SK; Wang SN Boron-based stimuli responsive materials. Chem. Soc. Rev 2019, 48, 3537–3549. - PubMed
- Inglis SR; Strieker M; Rydzik AM; Dessen A; Schofield CJ A boronic-acid-based probe for fluorescence polarization assays with penicillin binding proteins and b-lactamases. J. Anal. Biochem 2012, 420, 41–47. - PubMed
- Jin S; Zhu CY; Cheng YF; Li MY; Wang BH Synthesis and carbohydrate binding studies of fluorescent α-amidoboronic acids and the corresponding bisboronic acids. Bioorg. Med. Chem. Lett 2010, 18, 1449–1455. - PMC - PubMed
-
- Hall DG Ed.; Boronic Acids. Wiley-VCH: Weinheim, 2011.
-
- Xu NX; Liang H; Morken JP Copper-catalyzed stereospecific transformations of alkylboronic esters. J. Am. Chem. Soc 2022, 144, 11546–11552. - PMC - PubMed
- Liu X; Zhu Q; Chen D; Wang L; Jin L; Liu C Aminoazanium of DABCO: An amination reagent for alkyl and aryl pinacol boronates. Angew. Chem. Int. Ed 2020, 59, 2745–2749. - PubMed
- Namirembe S; Morken JP Reactions of organoboron compounds enabled by catalyst-promoted metalate shifts. Chem. Soc. Rev 2019, 48, 3464–3474. - PMC - PubMed
- Sandford C; Aggarwal VK Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun 2017, 53, 5481–5494. - PubMed
- Mlynarski SN; Karns AS; Morken JP Direct stereospecific amination of alkyl and aryl pinacol boronates. J. Am. Chem. Soc 2012, 134, 16449–16451. - PMC - PubMed
- Scott HK; Aggarwal VK Highly enantioselective synthesis of tertiary boronic esters and their stereospecific conversion to other functional groups and quaternary stereocentres. Chem. Eur. J 2011, 17, 13124–13132. - PubMed
- Matteson DS; Kim GY Asymmetric alkyldifluoroboranes and their use in secondary amine synthesis. Org. Lett 2002, 4, 2153–2155. - PubMed
- Matteson DS Functional group compatibilities in boronic ester chemistry. J. Organomet. Chem 1999, 581, 51–65.
-
-
For other recent reviews, see:
- Hu J; Ferger M; Shi Z; Marder TB Recent advances in asymmetric borylation by transition metal catalysis. Chem. Soc. Rev 2021, 50, 13129–13188. - PubMed
- Obligacion JV; Chirik PJ Earth-abundant transition metal catalysts for alkene hydrosilylation and hydroboration. Nature Rev. Chem 2018, 2, 15–34. - PMC - PubMed
- Burgess K; Ohlmeyer MJ Transition-metal promoted hydroborations of alkenes, emerging methodology for organic transformations. Chem. Rev 1991, 91, 1179–1191.
-
Grants and funding
LinkOut - more resources
Full Text Sources

