Outcomes in patients treated with frontline immune checkpoint inhibition (ICI) for advanced NSCLC with KRAS mutations and STK11/KEAP1 comutations across PD-L1 levels
- PMID: 38432028
- PMCID: PMC11194721
- DOI: 10.1016/j.lungcan.2024.107510
Outcomes in patients treated with frontline immune checkpoint inhibition (ICI) for advanced NSCLC with KRAS mutations and STK11/KEAP1 comutations across PD-L1 levels
Abstract
Introduction: In patients with advanced NSCLC (aNSCLC), the impact of KRAS mutations (m) and comutations with STK11 and KEAP1 on outcomes across different PD-L1 levels remains incompletely understood. We aimed to investigate the frequency of KRAS mutations and comutations across PD-L1 levels, and the association between these mutations and survival, stratified by PD-L1 expression.
Methods: We conducted a nationwide cohort study of patients diagnosed with aNSCLC between 2016 and 2021 treated with frontline (chemo)immunotherapy, who underwent molecular genotyping including KRAS, STK11, and KEAP1. Real-world overall survival (OS) and progression-free survival (rwPFS) were estimated using Kaplan-Meier methodology. Cox multivariable regressions were used to evaluate the association between KRASm and survival across different PD-L1 strata, and to assess whether the association between KRASm and survival differed by PD-L1 level. Finally, within subgroups defined by PD-L1 expression, we used interaction terms to assess whether co-mutations with STK11 and KEAP1 moderated the association between KRAS mutation and survival.
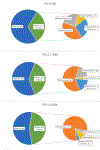
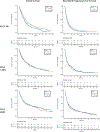
Results: Of our 2593-patient cohort, 982 (37.9 %) were KRASm and 1611 (62.1 %) KRASwt. KRASm were enriched in the PD-L1 ≥50 % cohort (334/743, 45 %), but within patients with KRASm, co-mutations with STK11 and KEAP1 were enriched in the PD-L1 0 % cohort. KRASm was associated with significantly worse OS in the PD-L1 0 % cohort compared to the PD-L1 ≥50 % cohort (P for interaction = 0.008). On adjusted analyses stratified by PD-L1, KRASm was associated with worse survival only in the PD-L1 0 % group (OS HR 1.46, p = 0.001). KEAP1 and STK11 comutations were most strongly associated with worse OS in the PD-L1 0 % subgroup; patients with triple KRASm/KEAPm/STK11m PD-L1 0 % NSCLC experienced the worst outcomes.
Conclusions: KRASm are associated with worse overall survival in PD-L1 negative NSCLC; however, this association is largely driven by comutations with STK11 and KEAP1, which are enriched in PD-L1 negative tumors.
Keywords: Immunotherapy; KRAS mutation; Non small cell lung cancer; Real world data.
Copyright © 2024 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Sun declares advisory board/consulting from Sanofi Genzyme, Regeneron, GenMab, Seagen, Bayer; and research support (clinical trials) from Blueprint, Seagen, IO Biotech, Erasca, Immunocore, and Abbvie. Dr. Borghaei declares research support (clinical trials) from BMS, Lilly, Amgen; advisory board/consulting from BMS, Lilly, Genentech, Pfizer, Merck, EMD-Serono, Boehringer Ingelheim, Astra Zeneca, Novartis, Genmab, Regeneron, BioNTech, Amgen, Axiom, PharmaMar, Takeda, Mirati, Daiichi, Guardant, Natera, Oncocyte, Beigene, iTEO, Jazz, Janssen, Puma, BerGenBio, Bayer, Iobiotech, Grid Therapeutics; DSMB for University of Pennsylvania: CAR T Program, Takeda, Incyte, Novartis, Springworks; honoraria from Amgen, Pfizer, Daiichi, Regeneron; stock options for Sonnetbio, Inspirna, Nucleai; and travel support from Amgen, BMS, Merck, Lilly, EMD-Serono, Genentech, Regeneron, Mirati. Dr. Bauman declares advisory board/consulting for EMD Serono and editorial support from Pfizer. Dr. Aggarwal declares grants from AstraZeneca, Genentech, Incyte, Loxo@Lilly, Macrogenics, Medimmune, and Merck Sharp & Dohme, and personal fees from Genentech, Lilly, Celgene Merck, AstraZeneca, Blueprint Genetics, Shionogi, Daiichi Sankyo/Astra Zeneca, Regeneron/Sanofi, Eisai, BeiGene, Turning Point, Pfizer, Janssen, Boehringer Ingelheim.
Figures
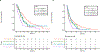
References
-
- Janne PA, Riely GJ, Gadgeel SM, et al.: Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRASG12C Mutation. New England Journal of Medicine 387:120–131, 2022 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

