VCD-induced menopause mouse model reveals reprogramming of hepatic metabolism
- PMID: 38432400
- PMCID: PMC10944007
- DOI: 10.1016/j.molmet.2024.101908
VCD-induced menopause mouse model reveals reprogramming of hepatic metabolism
Abstract
Objective: Menopause adversely impacts systemic energy metabolism and increases the risk of metabolic disease(s) including hepatic steatosis, but the mechanisms are largely unknown. Dosing female mice with vinyl cyclohexene dioxide (VCD) selectively causes follicular atresia in ovaries, leading to a murine menopause-like phenotype.
Methods: In this study, we treated female C57BL6/J mice with VCD (160 mg/kg i.p. for 20 consecutive days followed by verification of the lack of estrous cycling) to investigate changes in body composition, energy expenditure (EE), hepatic mitochondrial function, and hepatic steatosis across different dietary conditions.
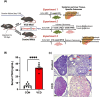
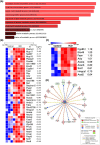
Results: VCD treatment induced ovarian follicular loss and increased follicle-stimulating hormone (FSH) levels in female mice, mimicking a menopause-like phenotype. VCD treatment did not affect body composition, or EE in mice on a low-fat diet (LFD) or in response to a short-term (1-week) high-fat, high sucrose diet (HFHS). However, the transition to a HFHS lowered cage activity in VCD mice. A chronic HFHS diet (16 weeks) significantly increased weight gain, fat mass, and hepatic steatosis in VCD-treated mice compared to HFHS-fed controls. In the liver, VCD mice showed suppressed hepatic mitochondrial respiration on LFD, while chronic HFHS resulted in compensatory increases in hepatic mitochondrial respiration. Also, liver RNA sequencing revealed that VCD promoted global upregulation of hepatic lipid/cholesterol synthesis pathways.
Conclusion: Our findings suggest that the VCD-induced menopause model compromises hepatic mitochondrial function and lipid/cholesterol homeostasis that sets the stage for HFHS diet-induced steatosis while also increasing susceptibility to obesity.
Keywords: Energy homeostasis; Estrogen; Liver metabolism; Menopause; Obesity; Sex hormones; Steatosis.
Published by Elsevier GmbH.
Conflict of interest statement
Declaration of competing interest None of the authors have any conflicts of interest to disclose.
Figures







Update of
-
VCD-induced menopause mouse model reveals reprogramming of hepatic metabolism.bioRxiv [Preprint]. 2023 Dec 14:2023.12.14.571644. doi: 10.1101/2023.12.14.571644. bioRxiv. 2023. Update in: Mol Metab. 2024 Apr;82:101908. doi: 10.1016/j.molmet.2024.101908. PMID: 38168213 Free PMC article. Updated. Preprint.
References
-
- Broekmans F.J., Soules M.R., Fauser B.C. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30(5):465–493. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

