SPAK inhibitor ZT-1a attenuates reactive astrogliosis and oligodendrocyte degeneration in a mouse model of vascular dementia
- PMID: 38433018
- PMCID: PMC10909630
- DOI: 10.1111/cns.14654
SPAK inhibitor ZT-1a attenuates reactive astrogliosis and oligodendrocyte degeneration in a mouse model of vascular dementia
Abstract
Background: Astrogliosis and white matter lesions (WML) are key characteristics of vascular contributions to cognitive impairment and dementia (VCID). However, the molecular mechanisms underlying VCID remain poorly understood. Stimulation of Na-K-Cl cotransport 1 (NKCC1) and its upstream kinases WNK (with no lysine) and SPAK (the STE20/SPS1-related proline/alanine-rich kinase) play a role in astrocytic intracellular Na+ overload, hypertrophy, and swelling. Therefore, in this study, we assessed the effect of SPAK inhibitor ZT-1a on pathogenesis and cognitive function in a mouse model of VCID induced by bilateral carotid artery stenosis (BCAS).
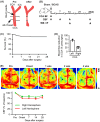
Methods: Following sham or BCAS surgery, mice were randomly assigned to receive either vehicle (DMSO) or SPAK inhibitor ZT-1a treatment regimen (days 14-35 post-surgery). Mice were then evaluated for cognitive functions by Morris water maze, WML by ex vivo MRI-DTI analysis, and astrogliosis/demyelination by immunofluorescence and immunoblotting.
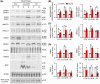
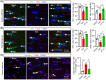
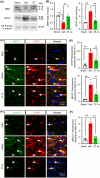
Results: Compared to sham control mice, BCAS-Veh mice exhibited chronic cerebral hypoperfusion and memory impairments, accompanied by significant MRI DTI-detected WML and oligodendrocyte (OL) death. Increased activation of WNK-SPAK-NKCC1-signaling proteins was detected in white matter tissues and in C3d+ GFAP+ cytotoxic astrocytes but not in S100A10+ GFAP+ homeostatic astrocytes in BCAS-Veh mice. In contrast, ZT-1a-treated BCAS mice displayed reduced expression and phosphorylation of NKCC1, decreased astrogliosis, OL death, and WML, along with improved memory functions.
Conclusion: BCAS-induced upregulation of WNK-SPAK-NKCC1 signaling contributes to white matter-reactive astrogliosis, OL death, and memory impairment. Pharmacological inhibition of the SPAK activity has therapeutic potential for alleviating pathogenesis and memory impairment in VCID.
Keywords: BCAS; NKCC1; VCID; ZT-1a; astrogliosis; vascular dementia.
© 2024 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Corriveau RA, Bosetti F, Emr M, et al. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol. 2016;36:281‐288. doi:10.1007/s10571-016-0334-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

