LncRNA Anxa10-203 enhances Mc1r mRNA stability to promote neuropathic pain by recruiting DHX30 in the trigeminal ganglion
- PMID: 38433184
- PMCID: PMC10910797
- DOI: 10.1186/s10194-024-01733-2
LncRNA Anxa10-203 enhances Mc1r mRNA stability to promote neuropathic pain by recruiting DHX30 in the trigeminal ganglion
Abstract
Background: Trigeminal nerve injury is one of the most serious complications in oral clinics, and the subsequent chronic orofacial pain is a consumptive disease. Increasing evidence demonstrates long non-coding RNAs (lncRNAs) play an important role in the pathological process of neuropathic pain. This study aims to explore the function and mechanism of LncRNA Anxa10-203 in the development of orofacial neuropathic pain.
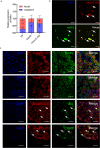
Methods: A mouse model of orofacial neuropathic pain was established by chronic constriction injury of the infraorbital nerve (CCI-ION). The Von Frey test was applied to evaluate hypersensitivity of mice. RT-qPCR and/or Western Blot were performed to analyze the expression of Anxa10-203, DHX30, and MC1R. Cellular localization of target genes was verified by immunofluorescence and RNA fluorescence in situ hybridization. RNA pull-down and RNA immunoprecipitation were used to detect the interaction between the target molecules. Electrophysiology was employed to assess the intrinsic excitability of TG neurons (TGNs) in vitro.
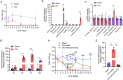
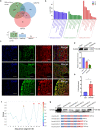
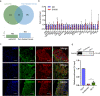
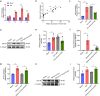
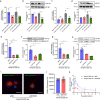
Results: Anxa10-203 was upregulated in the TG of CCI-ION mice, and knockdown of Anxa10-203 relieved neuropathic pain. Structurally, Anxa10-203 was located in the cytoplasm of TGNs. Mechanistically, Mc1r expression was positively correlated with Anxa10-203 and was identified as the functional target of Anxa10-203. Besides, Anxa10-203 recruited RNA binding protein DHX30 and formed the Anxa10-203/DHX30 complex to enhance the stability of Mc1r mRNA, resulting in the upregulation of MC1R, which contributed to the enhancement of the intrinsic activity of TGNs in vitro and orofacial neuropathic pain in vivo.
Conclusions: LncRNA Anxa10-203 in the TG played an important role in orofacial neuropathic pain and mediated mechanical allodynia in CCI-ION mice by binding with DHX30 to upregulate MC1R expression.
Keywords: DExH-box helicase 30; Long non-coding RNA; Melanocortin 1 receptor; Neuropathic pain; RNA-binding proteins; Trigeminal ganglion.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

