Induced pluripotent stem cell derived pericytes respond to mediators of proliferation and contractility
- PMID: 38433209
- PMCID: PMC10910734
- DOI: 10.1186/s13287-024-03671-x
Induced pluripotent stem cell derived pericytes respond to mediators of proliferation and contractility
Abstract
Background: Pericytes are multifunctional contractile cells that reside on capillaries. Pericytes are critical regulators of cerebral blood flow and blood-brain barrier function, and pericyte dysfunction may contribute to the pathophysiology of human neurological diseases including Alzheimers disease, multiple sclerosis, and stroke. Induced pluripotent stem cell (iPSC)-derived pericytes (iPericytes) are a promising tool for vascular research. However, it is unclear how iPericytes functionally compare to primary human brain vascular pericytes (HBVPs).
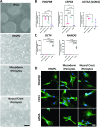
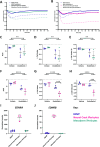
Methods: We differentiated iPSCs into iPericytes of either the mesoderm or neural crest lineage using established protocols. We compared iPericyte and HBVP morphologies, quantified gene expression by qPCR and bulk RNA sequencing, and visualised pericyte protein markers by immunocytochemistry. To determine whether the gene expression of neural crest iPericytes, mesoderm iPericytes or HBVPs correlated with their functional characteristics in vitro, we quantified EdU incorporation following exposure to the key pericyte mitogen, platelet derived growth factor (PDGF)-BB and, contraction and relaxation in response to the vasoconstrictor endothelin-1 or vasodilator adenosine, respectively.
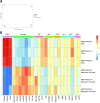
Results: iPericytes were morphologically similar to HBVPs and expressed canonical pericyte markers. However, iPericytes had 1864 differentially expressed genes compared to HBVPs, while there were 797 genes differentially expressed between neural crest and mesoderm iPericytes. Consistent with the ability of HBVPs to respond to PDGF-BB signalling, PDGF-BB enhanced and a PDGF receptor-beta inhibitor impaired iPericyte proliferation. Administration of endothelin-1 led to iPericyte contraction and adenosine led to iPericyte relaxation, of a magnitude similar to the response evoked in HBVPs. We determined that neural crest iPericytes were less susceptible to PDGFR beta inhibition, but responded most robustly to vasoconstrictive mediators.
Conclusions: iPericytes express pericyte-associated genes and proteins and, exhibit an appropriate physiological response upon exposure to a key endogenous mitogen or vasoactive mediators. Therefore, the generation of functional iPericytes would be suitable for use in future investigations exploring pericyte function or dysfunction in neurological diseases.
Keywords: Adenosine; Contractility; Endothelin-1; Human brain vascular pericytes (HBVPs); Induced pluripotent stem cells (iPSCs); Pericytes; Platelet-derived growth factor BB (PDGF-BB); Platelet-derived growth factor receptor β (PDGFRβ); Proliferation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Platelet-Derived Growth Factor Subunit B Signaling Promotes Pericyte Migration in Response to Loud Sound in the Cochlear Stria Vascularis.J Assoc Res Otolaryngol. 2018 Aug;19(4):363-379. doi: 10.1007/s10162-018-0670-z. Epub 2018 Jun 4. J Assoc Res Otolaryngol. 2018. PMID: 29869048 Free PMC article.
-
Contribution of brain pericytes to neuroinflammation following repetitive head trauma.J Inflamm (Lond). 2025 Mar 3;22(1):11. doi: 10.1186/s12950-025-00439-9. J Inflamm (Lond). 2025. PMID: 40033399 Free PMC article.
-
Human iPSC-derived pericyte-like cells carrying APP Swedish mutation overproduce beta-amyloid and induce cerebral amyloid angiopathy-like changes.Fluids Barriers CNS. 2024 Sep 27;21(1):78. doi: 10.1186/s12987-024-00576-y. Fluids Barriers CNS. 2024. PMID: 39334385 Free PMC article.
-
Prophylactic platelet transfusion for prevention of bleeding in patients with haematological disorders after chemotherapy and stem cell transplantation.Cochrane Database Syst Rev. 2012 May 16;2012(5):CD004269. doi: 10.1002/14651858.CD004269.pub3. Cochrane Database Syst Rev. 2012. PMID: 22592695 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
Cited by
-
Physiological Insights Into the Role of Pericytes in Spinal Cord Injury.J Cell Physiol. 2025 Jan;240(1):e31500. doi: 10.1002/jcp.31500. J Cell Physiol. 2025. PMID: 39757951 Free PMC article. Review.
-
Ameliorated cellular hallmarks of myotonic dystrophy in hybrid myotubes from patient and unaffected donor cells.Stem Cell Res Ther. 2024 Sep 15;15(1):302. doi: 10.1186/s13287-024-03913-y. Stem Cell Res Ther. 2024. PMID: 39278936 Free PMC article.
-
Endothelial TREM-1 mediates sepsis-induced blood‒brain barrier disruption and cognitive impairment via the PI3K/Akt pathway.J Neuroinflammation. 2025 May 27;22(1):142. doi: 10.1186/s12974-025-03469-5. J Neuroinflammation. 2025. PMID: 40426195 Free PMC article.
-
Mutated LRRK2 induces a reactive phenotype and alters migration in human iPSC-derived pericyte-like cells.Fluids Barriers CNS. 2024 Nov 18;21(1):92. doi: 10.1186/s12987-024-00592-y. Fluids Barriers CNS. 2024. PMID: 39551752 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

