Identification of the bacterial community that degrades phenanthrene sorbed to polystyrene nanoplastics using DNA-based stable isotope probing
- PMID: 38433255
- PMCID: PMC10909871
- DOI: 10.1038/s41598-024-55825-9
Identification of the bacterial community that degrades phenanthrene sorbed to polystyrene nanoplastics using DNA-based stable isotope probing
Abstract
In the Anthropocene, plastic pollution has become a new environmental biotope, the so-called plastisphere. In the oceans, nano- and micro-sized plastics are omnipresent and found in huge quantities throughout the water column and sediment, and their large surface area-to-volume ratio offers an excellent surface to which hydrophobic chemical pollutants (e.g. petrochemicals and POPs) can readily sorb to. Our understanding of the microbial communities that breakdown plastic-sorbed chemical pollutants, however, remains poor. Here, we investigated the formation of 500 nm and 1000 nm polystyrene (PS) agglomerations in natural seawater from a coastal environment, and we applied DNA-based stable isotope probing (DNA-SIP) with the 500 nm PS sorbed with isotopically-labelled phenanthrene to identify the bacterial members in the seawater community capable of degrading the hydrocarbon. Whilst we observed no significant impact of nanoplastic size on the microbial communities associated with agglomerates that formed in these experiments, these communities were, however, significantly different to those in the surrounding seawater. By DNA-SIP, we identified Arcobacteraceae, Brevundimonas, Comamonas, uncultured Comamonadaceae, Delftia, Sphingomonas and Staphylococcus, as well as the first member of the genera Acidiphilum and Pelomonas to degrade phenanthrene, and of the genera Aquabacterium, Paracoccus and Polymorphobacter to degrade a hydrocarbon. This work provides new information that feeds into our growing understanding on the fate of co-pollutants associated with nano- and microplastics in the ocean.
Keywords: DNA-SIP; Marine snow; Microplastics; Nanoplastics; Phenanthrene.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

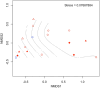

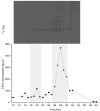

Similar articles
-
The Minderoo-Monaco Commission on Plastics and Human Health.Ann Glob Health. 2023 Mar 21;89(1):23. doi: 10.5334/aogh.4056. eCollection 2023. Ann Glob Health. 2023. PMID: 36969097 Free PMC article. Review.
-
Continuous generation and release of microplastics and nanoplastics from polystyrene by plastic-degrading marine bacteria.J Hazard Mater. 2024 Mar 5;465:133339. doi: 10.1016/j.jhazmat.2023.133339. Epub 2023 Dec 22. J Hazard Mater. 2024. PMID: 38150757
-
Agglomeration of nano- and microplastic particles in seawater by autochthonous and de novo-produced sources of exopolymeric substances.Mar Pollut Bull. 2018 May;130:258-267. doi: 10.1016/j.marpolbul.2018.03.039. Epub 2018 Apr 2. Mar Pollut Bull. 2018. PMID: 29866555
-
Interactions between phenanthrene and polystyrene micro/nano plastics: Implications for rice (Oryza sativa L.) toxicity.Environ Pollut. 2023 Nov 15;337:122360. doi: 10.1016/j.envpol.2023.122360. Epub 2023 Aug 19. Environ Pollut. 2023. PMID: 37604389
-
Adsorptive behavior of micro(nano)plastics through biochar: Co-existence, consequences, and challenges in contaminated ecosystems.Sci Total Environ. 2023 Jan 15;856(Pt 1):159097. doi: 10.1016/j.scitotenv.2022.159097. Epub 2022 Sep 28. Sci Total Environ. 2023. PMID: 36179840 Review.
References
-
- GESAMP. Fate and effects of microplastics in the marine environment: A global assessment (IMO/FAO/UNESCO-IOC/UNIDO/WMO/IAEA/UN/UNEP/UNDP joint group of experts on the scientific aspects of marine environmental protection). Rep. Stud. GESAMP (2015).
-
- Lyons MM, Ward JE, Uhlinger KR, Gast RJ, Smolowitz R. Lethal marine snow: Pathogen of bivalve mollusc concealed in marine aggregates. Limnol. Oceanogr. 2005;50:1983–1988. doi: 10.4319/lo.2005.50.6.1983. - DOI
-
- Ryan PG. Hazardous chemicals associated with plastics in the marine environment. In: Takada H, Karapanagioti HK, editors. The Handbook of Environmental Chemistry. Springer; 2016. pp. 235–266.
-
- Van Sebille E, et al. A global inventory of small floating plastic debris. Environ. Res. Lett. 2015;10:124006. doi: 10.1088/1748-9326/10/12/124006. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

