Somatic CAG repeat instability in intermediate alleles of the HTT gene and its potential association with a clinical phenotype
- PMID: 38433266
- PMCID: PMC11220145
- DOI: 10.1038/s41431-024-01546-6
Somatic CAG repeat instability in intermediate alleles of the HTT gene and its potential association with a clinical phenotype
Abstract
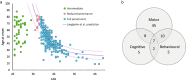
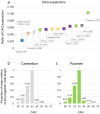
Huntington disease (HD) is a neurodegenerative disorder caused by ≥36 CAGs in the HTT gene. Intermediate alleles (IAs) (27-35 CAGs) are not considered HD-causing, but their potential association with neurocognitive symptoms remains controversial. As HTT somatic CAG expansion influences HD onset, we hypothesised that IAs are somatically unstable, and that somatic CAG expansion may drive phenotypic presentation in some IA carriers. We quantified HTT somatic CAG expansions by MiSeq sequencing in the blood DNA of 164 HD subjects and 191 IA (symptomatic and control) carriers, and in the brain DNA of a symptomatic 33 CAG carrier. We also performed genotype-phenotype analysis. The phenotype of symptomatic IA carriers was characterised by motor (85%), cognitive (27%) and/or behavioural (29%) signs, with a late (58.7 ± 18.6 years), but not CAG-dependent, age at onset. IAs displayed somatic expansion that were CAG and age-dependent in blood DNA, with 0.4% and 0.01% of DNA molecules expanding by CAG and year, respectively. Somatic expansions of +1 and +2 CAGs were detected in the brain of the individual with 33 CAGs, with the highest expansion frequency in the putamen (10.3%) and the lowest in the cerebellum (4.8%). Somatic expansion in blood DNA was not different in symptomatic vs. control IA carriers. In conclusion, we show that HTT IAs are somatically unstable, but we found no association with HD-like phenotypes. It is plausible, however, that some IAs, close to the HD pathological threshold and with a predisposing genetic background, could manifest with neurocognitive symptoms.
© 2024. The Author(s).
Conflict of interest statement
Within the last 5 years, DGM has been a scientific consultant and/or received an honoraria/stock options/grants from AMO Pharma, Charles River, LoQus23, Triplet Therapeutics, Ono Pharmaceuticals, Rgenta Therapeutics, Novartis, Dyne and Vertex Pharmaceuticals. DGM also had research contracts with AMO Pharma and Vertex Pharmaceuticals.
Figures


Comment in
-
Comment on: "Somatic CAG repeat instability in intermediate alleles of the HTT gene and its potential association with a clinical phenotype" by Ruiz de Sabando et al.Eur J Hum Genet. 2024 Jul;32(7):745-746. doi: 10.1038/s41431-024-01587-x. Epub 2024 Mar 7. Eur J Hum Genet. 2024. PMID: 38448560 Free PMC article. No abstract available.
References
-
- Rubinsztein DC, Leggo J, Coles R, Almqvist E, Biancalana V, Cassiman JJ, et al. Phenotypic characterization of individuals with 30-40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36-39 repeats. Am J Hum Genet. 1996;59:16–22. - PMC - PubMed
-
- Apolinário TA, Paiva CLA, Agostinho LA. REVIEW-ARTICLE Intermediate alleles of Huntington’s disease HTT gene in different populations worldwide: a systematic review. Genet Mol Res. 2017;16. 10.4238/gmr16029648. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

