Host genotype-specific rhizosphere fungus enhances drought resistance in wheat
- PMID: 38433268
- PMCID: PMC10910722
- DOI: 10.1186/s40168-024-01770-8
Host genotype-specific rhizosphere fungus enhances drought resistance in wheat
Erratum in
-
Correction: Host genotype-specific rhizosphere fungus enhances drought resistance in wheat.Microbiome. 2024 Mar 23;12(1):61. doi: 10.1186/s40168-024-01794-0. Microbiome. 2024. PMID: 38520015 Free PMC article. No abstract available.
Abstract
Background: The severity and frequency of drought are expected to increase substantially in the coming century and dramatically reduce crop yields. Manipulation of rhizosphere microbiomes is an emerging strategy for mitigating drought stress in agroecosystems. However, little is known about the mechanisms underlying how drought-resistant plant recruitment of specific rhizosphere fungi enhances drought adaptation of drought-sensitive wheats. Here, we investigated microbial community assembly features and functional profiles of rhizosphere microbiomes related to drought-resistant and drought-sensitive wheats by amplicon and shotgun metagenome sequencing techniques. We then established evident linkages between root morphology traits and putative keystone taxa based on microbial inoculation experiments. Furthermore, root RNA sequencing and RT-qPCR were employed to explore the mechanisms how rhizosphere microbes modify plant response traits to drought stresses.
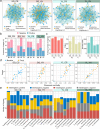
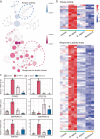
Results: Our results indicated that host plant signature, plant niche compartment, and planting site jointly contribute to the variation of soil microbiome assembly and functional adaptation, with a relatively greater effect of host plant signature observed for the rhizosphere fungi community. Importantly, drought-resistant wheat (Yunhan 618) possessed more diverse bacterial and fungal taxa than that of the drought-sensitive wheat (Chinese Spring), particularly for specific fungal species. In terms of microbial interkingdom association networks, the drought-resistant variety possessed more complex microbial networks. Metagenomics analyses further suggested that the enriched rhizosphere microbiomes belonging to the drought-resistant cultivar had a higher investment in energy metabolism, particularly in carbon cycling, that shaped their distinctive drought tolerance via the mediation of drought-induced feedback functional pathways. Furthermore, we observed that host plant signature drives the differentiation in the ecological role of the cultivable fungal species Mortierella alpine (M. alpina) and Epicoccum nigrum (E. nigrum). The successful colonization of M. alpina on the root surface enhanced the resistance of wheats in response to drought stresses via activation of drought-responsive genes (e.g., CIPK9 and PP2C30). Notably, we found that lateral roots and root hairs were significantly suppressed by co-colonization of a drought-enriched fungus (M. alpina) and a drought-depleted fungus (E. nigrum).
Conclusions: Collectively, our findings revealed host genotypes profoundly influence rhizosphere microbiome assembly and functional adaptation, as well as it provides evidence that drought-resistant plant recruitment of specific rhizosphere fungi enhances drought tolerance of drought-sensitive wheats. These findings significantly underpin our understanding of the complex feedbacks between plants and microbes during drought, and lay a foundation for steering "beneficial keystone biome" to develop more resilient and productive crops under climate change. Video Abstract.
Keywords: Drought; Microbiome; Multi-omics; Rhizosphere; Wheat.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Plant domestication shapes rhizosphere microbiome assembly and metabolic functions.Microbiome. 2023 Mar 31;11(1):70. doi: 10.1186/s40168-023-01513-1. Microbiome. 2023. PMID: 37004105 Free PMC article.
-
Enrichment of beneficial rhizosphere microbes in Chinese wheat yellow mosaic virus-resistant cultivars.Appl Microbiol Biotechnol. 2021 Dec;105(24):9371-9383. doi: 10.1007/s00253-021-11666-4. Epub 2021 Nov 12. Appl Microbiol Biotechnol. 2021. PMID: 34767052
-
Drought-induced assembly of rhizosphere mycobiomes shows beneficial effects on plant growth.mSystems. 2024 Jul 23;9(7):e0035424. doi: 10.1128/msystems.00354-24. Epub 2024 Jun 6. mSystems. 2024. PMID: 38842321 Free PMC article.
-
Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation.Microbiol Res. 2021 Jan;242:126626. doi: 10.1016/j.micres.2020.126626. Epub 2020 Oct 18. Microbiol Res. 2021. PMID: 33189069 Review.
-
Harnessing rhizosphere microbiomes for drought-resilient crop production.Science. 2020 Apr 17;368(6488):270-274. doi: 10.1126/science.aaz5192. Science. 2020. PMID: 32299947 Review.
Cited by
-
Different Flooding Conditions Affected Microbial Diversity in Riparian Zone of Huihe Wetland.Microorganisms. 2025 Jan 13;13(1):154. doi: 10.3390/microorganisms13010154. Microorganisms. 2025. PMID: 39858922 Free PMC article.
-
Correction: Host genotype-specific rhizosphere fungus enhances drought resistance in wheat.Microbiome. 2024 Mar 23;12(1):61. doi: 10.1186/s40168-024-01794-0. Microbiome. 2024. PMID: 38520015 Free PMC article. No abstract available.
-
Bikaverin as a molecular weapon: enhancing Fusarium oxysporum pathogenicity in bananas via rhizosphere microbiome manipulation.Microbiome. 2025 Apr 29;13(1):107. doi: 10.1186/s40168-025-02109-7. Microbiome. 2025. PMID: 40301992 Free PMC article.
-
Mitigating Water Stress in Plants with Beneficial Bacteria: Effects on Growth and Rhizosphere Bacterial Communities.Int J Mol Sci. 2025 Feb 10;26(4):1467. doi: 10.3390/ijms26041467. Int J Mol Sci. 2025. PMID: 40003931 Free PMC article.
-
Study on the Biological Characteristics of Dark Septate Endophytes under Drought and Cadmium Stress and Their Effects on Regulating the Stress Resistance of Astragalus membranaceus.J Fungi (Basel). 2024 Jul 16;10(7):491. doi: 10.3390/jof10070491. J Fungi (Basel). 2024. PMID: 39057377 Free PMC article.
References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

