Aiouea padiformis extract exhibits anti-inflammatory effects by inhibiting the ATPase activity of NLRP3
- PMID: 38433281
- PMCID: PMC10909851
- DOI: 10.1038/s41598-024-55651-z
Aiouea padiformis extract exhibits anti-inflammatory effects by inhibiting the ATPase activity of NLRP3
Abstract
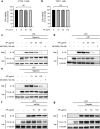
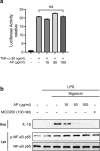
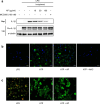
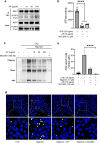
Inflammation is implicated as a cause in many diseases. Most of the anti-inflammatory agents in use are synthetic and there is an unmet need for natural substance-derived anti-inflammatory agents with minimal side effects. Aiouea padiformis belongs to the Lauraceae family and is primarily found in tropical regions. While some members of the Aiouea genus are known to possess anti-inflammatory properties, the anti-inflammatory properties of Aiouea padiformis extract (AP) have not been investigated. In this study, we aimed to examine the anti-inflammatory function of AP through the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome and elucidate the underlying mechanisms. Treatment with AP inhibited the secretion of interleukin-1 beta (IL-1β) mediated by NLRP3 inflammasome in J774A.1 and THP-1 cells without affecting the viability. In addition, AP treatment did not influence NF-κB signaling, potassium efflux, or intracellular reactive oxygen species (ROS) production-all of which are associated with NLRP3 inflammasome activation. However, intriguingly, AP treatment significantly reduced the ATPase activity of NLRP3, leading to the inhibition of ASC oligomerization and speck formation. Consistent with cellular experiments, the anti-inflammatory property of AP in vivo was also evaluated using an LPS-induced inflammation model in zebrafish, demonstrating that AP hinders NLRP3 inflammasome activation.
Keywords: Aiouea padiformis; Anti-inflammation; Lauraceae; NLRP3 inflammasome; Plant extracts.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Guarea microcarpa C. DC. extract inhibits NLRP3 inflammasome by suppressing its ATPase activity.J Ethnopharmacol. 2024 Apr 6;323:117711. doi: 10.1016/j.jep.2024.117711. Epub 2024 Jan 3. J Ethnopharmacol. 2024. PMID: 38176663
-
Anti-inflammatory effect of Trichospira verticillata via suppression of the NLRP3 inflammasome in neutrophilic asthma.J Cell Mol Med. 2024 Apr;28(8):e18356. doi: 10.1111/jcmm.18356. J Cell Mol Med. 2024. PMID: 38668995 Free PMC article.
-
The ethanolic extract of Artemisia anomala exerts anti-inflammatory effects via inhibition of NLRP3 inflammasome.Phytomedicine. 2022 Jul 20;102:154163. doi: 10.1016/j.phymed.2022.154163. Epub 2022 May 10. Phytomedicine. 2022. PMID: 35597027
-
Recent advances in the NEK7-licensed NLRP3 inflammasome activation: Mechanisms, role in diseases and related inhibitors.J Autoimmun. 2020 Sep;113:102515. doi: 10.1016/j.jaut.2020.102515. Epub 2020 Jul 20. J Autoimmun. 2020. PMID: 32703754 Review.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
Cited by
-
NLRP3 inflammasome and pyroptosis in cardiovascular diseases and exercise intervention.Front Pharmacol. 2024 Apr 12;15:1368835. doi: 10.3389/fphar.2024.1368835. eCollection 2024. Front Pharmacol. 2024. PMID: 38681198 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

