Increased oxidized low-density lipoprotein in mice exposed to a high-fat diet impaired spermatogenesis by inhibiting testosterone synthesis via the Klk1bs/Eid3 pathway
- PMID: 38433441
- PMCID: PMC10909978
- DOI: 10.1002/ctm2.1603
Increased oxidized low-density lipoprotein in mice exposed to a high-fat diet impaired spermatogenesis by inhibiting testosterone synthesis via the Klk1bs/Eid3 pathway
Conflict of interest statement
The authors declare no conflict of interest.
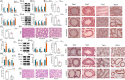
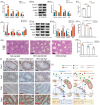
Figures


References
-
- Matsui H, Takahashi T. Mouse testicular Leydig cells express Klk21, a tissue kallikrein that cleaves fibronectin and IGF‐binding protein‐3. Endocrinology. 2001;142:4918‐4929. - PubMed
-
- Matsui H, Moriyama A, Takahashi T. Cloning and characterization of mouse klk27, a novel tissue kallikrein expressed in testicular Leydig cells and exhibiting chymotrypsin‐like specificity. Eur J Biochem. 2000;267:6858‐6865. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 32172726/National Natural Science Foundation of China
- 32272872/National Natural Science Foundation of China
- 20210202103NC/Key Research and Development Program of Jilin Province
- 20210202048NC/Key Research and Development Program of Jilin Province
- 20190301008NY/Key Research and Development Program of Jilin Province
LinkOut - more resources
Full Text Sources
