From pain to tumor immunity: influence of peripheral sensory neurons in cancer
- PMID: 38433844
- PMCID: PMC10905387
- DOI: 10.3389/fimmu.2024.1335387
From pain to tumor immunity: influence of peripheral sensory neurons in cancer
Abstract
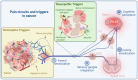
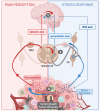
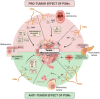
The nervous and immune systems are the primary sensory interfaces of the body, allowing it to recognize, process, and respond to various stimuli from both the external and internal environment. These systems work in concert through various mechanisms of neuro-immune crosstalk to detect threats, provide defense against pathogens, and maintain or restore homeostasis, but can also contribute to the development of diseases. Among peripheral sensory neurons (PSNs), nociceptive PSNs are of particular interest. They possess a remarkable capability to detect noxious stimuli in the periphery and transmit this information to the brain, resulting in the perception of pain and the activation of adaptive responses. Pain is an early symptom of cancer, often leading to its diagnosis, but it is also a major source of distress for patients as the disease progresses. In this review, we aim to provide an overview of the mechanisms within tumors that are likely to induce cancer pain, exploring a range of factors from etiological elements to cellular and molecular mediators. In addition to transmitting sensory information to the central nervous system, PSNs are also capable, when activated, to produce and release neuropeptides (e.g., CGRP and SP) from their peripheral terminals. These neuropeptides have been shown to modulate immunity in cases of inflammation, infection, and cancer. PSNs, often found within solid tumors, are likely to play a significant role in the tumor microenvironment, potentially influencing both tumor growth and anti-tumor immune responses. In this review, we discuss the current state of knowledge about the degree of sensory innervation in tumors. We also seek to understand whether and how PSNs may influence the tumor growth and associated anti-tumor immunity in different mouse models of cancer. Finally, we discuss the extent to which the tumor is able to influence the development and functions of the PSNs that innervate it.
Keywords: cancer; immune cells; neuropeptides; nociceptors; pain; peripheral sensory neurons; tumor immunity; tumor microenvironment.
Copyright © 2024 Mardelle, Bretaud, Daher and Feuillet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
[Pathophysiology of low back pain and the transition to the chronic state - experimental data and new concepts].Schmerz. 2001 Dec;15(6):413-7. doi: 10.1007/s004820100002. Schmerz. 2001. PMID: 11793144 German.
-
Profiling of how nociceptor neurons detect danger - new and old foes.J Intern Med. 2019 Sep;286(3):268-289. doi: 10.1111/joim.12957. Epub 2019 Jul 29. J Intern Med. 2019. PMID: 31282104 Review.
-
Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation.Trends Immunol. 2017 Jan;38(1):5-19. doi: 10.1016/j.it.2016.10.001. Epub 2016 Oct 25. Trends Immunol. 2017. PMID: 27793571 Free PMC article. Review.
-
Role of specialized sensory neuron subtypes in modulating peripheral immune responses.Immunity. 2025 May 13;58(5):1161-1174. doi: 10.1016/j.immuni.2025.04.008. Epub 2025 May 4. Immunity. 2025. PMID: 40324383 Review.
-
Cutaneous Neuroimmune Interactions in Peripheral Neuropathic Pain States.Front Immunol. 2021 Apr 12;12:660203. doi: 10.3389/fimmu.2021.660203. eCollection 2021. Front Immunol. 2021. PMID: 33912189 Free PMC article. Review.
Cited by
-
Repetitive Transcranial Magnetic Stimulation: Is it an Effective Treatment for Cancer Pain?Pain Ther. 2025 Feb;14(1):47-66. doi: 10.1007/s40122-024-00679-2. Epub 2024 Nov 17. Pain Ther. 2025. PMID: 39551863 Free PMC article. Review.
-
Intra-tumoural RAMP1+ B cells promote resistance to neoadjuvant anti-PD-1-based therapy in oesophageal squamous cell carcinoma.Immunother Adv. 2025 Mar 22;5(1):ltaf012. doi: 10.1093/immadv/ltaf012. eCollection 2025. Immunother Adv. 2025. PMID: 40385640 Free PMC article.
-
Neuroscience in peripheral cancers: tumors hijacking nerves and neuroimmune crosstalk.MedComm (2020). 2024 Oct 31;5(11):e784. doi: 10.1002/mco2.784. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39492832 Free PMC article. Review.
-
Role of TRP Channels in Cancer-Induced Bone Pain.Int J Mol Sci. 2025 Jan 30;26(3):1229. doi: 10.3390/ijms26031229. Int J Mol Sci. 2025. PMID: 39940997 Free PMC article. Review.
-
Sensory neurotransmission and pain in solid tumor progression.Trends Cancer. 2025 Apr;11(4):309-320. doi: 10.1016/j.trecan.2025.01.003. Epub 2025 Jan 30. Trends Cancer. 2025. PMID: 39884880 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

