Protocol to evaluate the efficacy and safety of tolvaptan in patients with refractory ascites after liver resection: an open-label, single-arm phase I/II study
- PMID: 38433869
- PMCID: PMC10905494
- DOI: 10.1097/SP9.0000000000000015
Protocol to evaluate the efficacy and safety of tolvaptan in patients with refractory ascites after liver resection: an open-label, single-arm phase I/II study
Abstract
Background: In patients with chronic liver diseases such as cirrhosis, massive ascites after hepatic resection is the cause of prolonged hospitalization and worsening prognosis. Recently, the efficacy of tolvaptan in refractory ascites has been reported; however, there are no reports on the efficacy or safety of tolvaptan for refractory ascites after hepatic resection. This study aims to evaluate the efficacy of early administration of tolvaptan in patients with refractory ascites after hepatic resection.
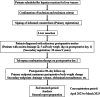
Materials and methods: This is an open-label, single-arm phase I/II study. This study subject will comprise patients scheduled for hepatic resection of a liver tumor. Patients with refractory ascites after hepatic resection (drainage volume on postoperative day 1 ≥5 ml/body weight 1 kg/day) will be treated with tolvaptan. The primary endpoint will include the maximum change in body weight after hepatic resection relative to the preoperative baseline. The secondary endpoints will include drainage volume, abdominal circumference, urine output, postoperative complication rate (heart failure and respiratory failure), number of days required for postoperative weight gain because of ascites to decrease to preoperative weight, change in improvement of postoperative pleural effusion, total amount of albumin or fresh frozen plasma transfusion, type and amount of diuretics used, and postoperative hospitalization days.
Conclusion: This trial will evaluate the efficacy and safety of tolvaptan prophylaxis for refractory ascites after hepatic resection. As there are no reports demonstrating the efficacy of tolvaptan prophylaxis for refractory ascites after hepatic resection, the authors expect that these findings will lead to future phase III trials and provide valuable indications for the selection of treatments for refractory postoperative ascites.
Keywords: hepatic resection; postoperative ascites; refractory ascites; tolvaptan.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare that they have no competing interests in this study.
References
-
- Ishizawa T, Hasegawa K, Kokudo N, et al. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg 2009;144:46–51. - PubMed
-
- Famularo S, Donadon M, Cipriani F, et al. Hepatocellular carcinoma surgical and oncological trends in a national multicentric population: the HERCOLES experience. Updates Surg 2020;72:399–411. - PubMed
-
- Kikuchi Y, Hiroshima Y, Matsuo K, et al. A randomized clinical trial of preoperative administration of branched-chain amino acids to prevent postoperative ascites in patients with liver resection for hepatocellular carcinoma. Ann Surg Oncol 2016;23:3727–3735. - PubMed
-
- Itoh S, Morita K, Ueda S, et al. Long-term results of hepatic resection combined with intraoperative local ablation therapy for patients with multinodular hepatocellular carcinomas. Annals Surg Oncol 2009;16:3299–3307. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials