The dabABC operon is a marker of C4-alkylated monobactam biosynthesis and responsible for (2S, 3R)-diaminobutyrate production
- PMID: 38433893
- PMCID: PMC10906522
- DOI: 10.1016/j.isci.2024.109202
The dabABC operon is a marker of C4-alkylated monobactam biosynthesis and responsible for (2S, 3R)-diaminobutyrate production
Erratum in
-
Erratum: The dabABC operon is a marker of C4-alkylated monobactam biosynthesis and responsible for (2S,3R)-diaminobutyrate production.iScience. 2024 Apr 3;27(4):109572. doi: 10.1016/j.isci.2024.109572. eCollection 2024 Apr 19. iScience. 2024. PMID: 38600972 Free PMC article.
Abstract
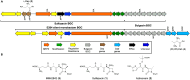
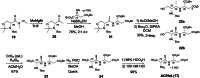
Non-ribosomal peptide synthetases (NRPSs) assemble metabolites of medicinal and commercial value. Both serine and threonine figure prominently in these processes and separately can be converted to the additional NRPS building blocks 2,3-diaminopropionate (Dap) and 2,3-diaminobutyrate (Dab). Here we bring extensive bioinformatics, in vivo and in vitro experimentation to compose a unified view of the biosynthesis of these widely distributed non-canonical amino acids that both derive by pyridoxal-mediated β-elimination of the activated O-phosphorylated substrates followed by β-addition of an amine donor. By examining monobactam biosynthesis in Pseudomonas and in Burkholderia species where it is silent, we show that (2S,3R)-Dab synthesis depends on an l-threonine kinase (DabA), a β-replacement reaction with l-aspartate (DabB) and an argininosuccinate lyase-like protein (DabC). The growing clinical importance of monobactams to both withstand Ambler Class B metallo-β-lactamases and retain their antibiotic activity make reprogrammed precursor and NRPS synthesis of modified monobactams a feasible and attractive goal.
Keywords: Chemistry.
© 2024 The Author(s).
Conflict of interest statement
The authors have no conflicts of interest.
Figures






References
-
- Centers for Disease Control and Prevention (U.S.) National Center for Emerging Zoonotic and Infectious Diseases (U.S.) National Center for HIV/AIDS, V.H., STD, and TB Prevention (U.S.) National Center for Immunization and Respiratory Diseases (U.S.) Antibiotic resistance threats in the United States, 2013. April 23, 2013. 2013. https://stacks.cdc.gov/view/cdc/20705
-
- World Health Organization . European Centre for Disease Prevention and Control and World Health Organization; 2023. Antimicrobial resistance surveillance in Europe - 2021 data.
-
- Hinchliffe P., Moreno D.M., Rossi M.A., Mojica M.F., Martinez V., Villamil V., Spellberg B., Drusano G.L., Banchio C., Mahler G., et al. 2-Mercaptomethyl Thiazolidines (MMTZs) Inhibit All Metallo-β-Lactamase Classes by Maintaining a Conserved Binding Mode. ACS Infect. Dis. 2021;7:2697–2706. doi: 10.1021/acsinfecdis.1c00194. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

