Homozygous ALS-linked mutations in TARDBP/TDP-43 lead to hypoactivity and synaptic abnormalities in human iPSC-derived motor neurons
- PMID: 38433895
- PMCID: PMC10905001
- DOI: 10.1016/j.isci.2024.109166
Homozygous ALS-linked mutations in TARDBP/TDP-43 lead to hypoactivity and synaptic abnormalities in human iPSC-derived motor neurons
Abstract
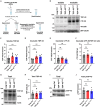
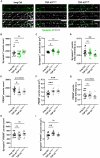
Cytoplasmic mislocalization and aggregation of the RNA-binding protein TDP-43 is a pathological hallmark of the motor neuron (MN) disease amyotrophic lateral sclerosis (ALS). Furthermore, while mutations in TARDBP (encoding TDP-43) have been associated with ALS, the pathogenic consequences of these mutations remain poorly understood. Using CRISPR-Cas9, we engineered two homozygous knock-in induced pluripotent stem cell lines carrying mutations in TARDBP encoding TDP-43A382T and TDP-43G348C, two common yet understudied ALS TDP-43 variants. Motor neurons (MNs) differentiated from knock-in iPSCs had normal viability and displayed no significant changes in TDP-43 subcellular localization, phosphorylation, solubility, or aggregation compared with isogenic control MNs. However, our results highlight synaptic impairments in both TDP-43A382T and TDP-43G348C MN cultures, as reflected in synapse abnormalities and alterations in spontaneous neuronal activity. Collectively, our findings suggest that MN dysfunction may precede the occurrence of TDP-43 pathology and neurodegeneration in ALS and further implicate synaptic and excitability defects in the pathobiology of this disease.
Keywords: Cellular neuroscience; Molecular neuroscience.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Transcriptome-based screening in TARDBP/TDP-43 knock-in motor neurons identifies the NEDD8-activating enzyme inhibitor MLN4924.Sci Rep. 2025 Aug 5;15(1):28555. doi: 10.1038/s41598-025-12147-8. Sci Rep. 2025. PMID: 40764342 Free PMC article.
-
Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo.Hum Mol Genet. 2010 Feb 15;19(4):671-83. doi: 10.1093/hmg/ddp534. Epub 2009 Dec 3. Hum Mol Genet. 2010. PMID: 19959528
-
TARDBP pathogenic mutations increase cytoplasmic translocation of TDP-43 and cause reduction of endoplasmic reticulum Ca²⁺ signaling in motor neurons.Neurobiol Dis. 2015 Mar;75:64-77. doi: 10.1016/j.nbd.2014.12.010. Epub 2014 Dec 17. Neurobiol Dis. 2015. PMID: 25526708
-
Mutations in TDP-43 link glycine-rich domain functions to amyotrophic lateral sclerosis.Hum Mol Genet. 2009 Oct 15;18(R2):R156-62. doi: 10.1093/hmg/ddp303. Hum Mol Genet. 2009. PMID: 19808791 Free PMC article. Review.
-
The molecular link between inefficient GluA2 Q/R site-RNA editing and TDP-43 pathology in motor neurons of sporadic amyotrophic lateral sclerosis patients.Brain Res. 2014 Oct 10;1584:28-38. doi: 10.1016/j.brainres.2013.12.011. Epub 2013 Dec 16. Brain Res. 2014. PMID: 24355598 Review.
Cited by
-
Emerging perspectives of synaptic biomarkers in ALS and FTD.Front Mol Neurosci. 2024 Jan 5;16:1279999. doi: 10.3389/fnmol.2023.1279999. eCollection 2023. Front Mol Neurosci. 2024. PMID: 38249293 Free PMC article. Review.
-
Loss of TDP-43 induces synaptic dysfunction that is rescued by UNC13A splice-switching ASOs.bioRxiv [Preprint]. 2024 Jun 24:2024.06.20.599684. doi: 10.1101/2024.06.20.599684. bioRxiv. 2024. PMID: 38979232 Free PMC article. Preprint.
-
TDP43 is a newly identified substrate for PS1, enhancing the expression of APP following cleavage.Cell Death Discov. 2025 Feb 23;11(1):76. doi: 10.1038/s41420-025-02340-z. Cell Death Discov. 2025. PMID: 39988698 Free PMC article.
-
Cytoplasmic TDP-43 leads to early functional impairments without neurodegeneration in a Serotonergic Neuron-Specific C. elegans Model.bioRxiv [Preprint]. 2025 Jul 31:2025.07.30.667669. doi: 10.1101/2025.07.30.667669. bioRxiv. 2025. PMID: 40766632 Free PMC article. Preprint.
-
Induced Pluripotent Stem Cells-Based Regenerative Therapies in Treating Human Aging-Related Functional Decline and Diseases.Cells. 2025 Apr 21;14(8):619. doi: 10.3390/cells14080619. Cells. 2025. PMID: 40277944 Free PMC article. Review.
References
-
- Kenna K.P., McLaughlin R.L., Byrne S., Elamin M., Heverin M., Kenny E.M., Cormican P., Morris D.W., Donaghy C.G., Bradley D.G., Hardiman O. Delineating the genetic heterogeneity of ALS using targeted high-throughput sequencing. J. Med. Genet. 2013;50:776–783. doi: 10.1136/jmedgenet-2013-101795. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

