Preparation of biocompatibility coating on magnesium alloy surface by sodium alginate and carboxymethyl chitosan hydrogel
- PMID: 38433902
- PMCID: PMC10904997
- DOI: 10.1016/j.isci.2024.109197
Preparation of biocompatibility coating on magnesium alloy surface by sodium alginate and carboxymethyl chitosan hydrogel
Abstract
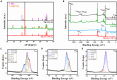
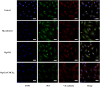
Magnesium alloy is an excellent material for biodegradable cerebrovascular stents. However, the rapid degradation rate of magnesium alloy will make stent unstable. To improve the biocompatibility of magnesium alloy, in this study, biodegradable sodium alginate and carboxymethyl chitosan (SA/CMCS) was used to coat onto hydrothermally treated the surface of magnesium alloy by a dipping coating method. The results show that the SA/CMCS coating facilitates the growth, proliferation, and migration of endothelial cells and promotes neovascularization. Moreover, the SA/CMCS coating suppresses macrophage activation while promoting their transformation into M2 type macrophages. Overall, the SA/CMCS coating demonstrates positive effects on the safety and biocompatibility of magnesium alloy after implantation, and provide a promising therapy for the treatment of intracranial atherosclerotic stenosis in the future.
Keywords: Applied sciences; Biomaterials.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures










References
-
- Liu M., Wang J., Zhu S., Zhang Y., Sun Y., Wang L., Guan S. Corrosion fatigue of the extruded Mg–Zn–Y–Nd alloy in simulated body fluid. J. Magnesium Alloys. 2020;8:231–240. doi: 10.1016/j.jma.2019.09.009. - DOI
LinkOut - more resources
Full Text Sources

