A chitosan-based antibacterial hydrogel with injectable and self-healing capabilities
- PMID: 38433964
- PMCID: PMC10902234
- DOI: 10.1007/s42995-023-00211-z
A chitosan-based antibacterial hydrogel with injectable and self-healing capabilities
Abstract
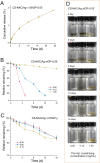
The presence of bacteria directly affects wound healing. Chitosan-based hydrogel biomaterials are a solution as they offer advantages for wound-healing applications due to their strong antimicrobial properties. Here, a double-cross-linking chitosan-based hydrogel with antibacterial, self-healing, and injectable properties is reported. Thiolated chitosan was successfully prepared, and the thiolated chitosan molecules were cross-linked by Ag-S coordination to form a supramolecular hydrogel. Subsequently, the amine groups in the thiolated chitosan covalently cross-linked with genipin to further promote hydrogel formation. In vitro experimental results indicate that hydrogel can release Ag+ over an extended time, achieving an antibacterial rate of over 99% against Escherichia coli and Staphylococcus aureus. Due to the reversible and dynamic feature of Ag-S coordination, an antibacterial hydrogel exhibited injectable and self-healing capabilities. Additionally, the hydrogel showed excellent biocompatibility and biodegradability.
Supplementary information: The online version contains supplementary material available at 10.1007/s42995-023-00211-z.
Keywords: Antibacterial; Chitosan; Cross-linking; Hydrogel; Silver ion.
© The Author(s) 2023.
Conflict of interest statement
Conflict of interestThe authors declare they have no conflict of interest.
Figures





References
-
- Alshameri AW, Owais M. Antibacterial and cytotoxic potency of the plant-mediated synthesis of metallic nanoparticles Ag NPs and ZnO NPs: a review. OpenNano. 2022;8:100077. doi: 10.1016/j.onano.2022.100077. - DOI
LinkOut - more resources
Full Text Sources

