Acquired hemophilia A: a single-center study of 165 patients
- PMID: 38433975
- PMCID: PMC10907205
- DOI: 10.1016/j.rpth.2024.102318
Acquired hemophilia A: a single-center study of 165 patients
Abstract
Background: Acquired hemophilia A (AHA) is a rare hemorrhagic disorder caused by factor (F)VIII inhibitors. The diagnosis and management of AHA remains challenging because of its rarity and heterogeneity.
Objectives: To analyze the characteristics of AHA to enhance our understanding of this disease and identify effective treatment strategies. To analyze the characteristics of AHA to enhance our understanding of this disease and identify effective treatment strategies.
Methods: Clinical features of 165 patients with AHA from a single center between July 1997 and December 2021 were retrospectively analyzed.
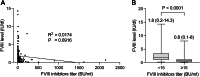
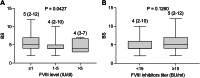
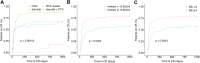
Results: The median age of patients at diagnosis was 45 years. The median time to diagnosis was 30 days. All 165 patients experienced bleeding, with a median bleeding score (BS) of 4 (range, 2-12). Hemostatic therapy was administered to 129 (78.2%) patients. Bleeding control was achieved in 80.0% of patients who received prothrombin complex concentrate and in 92.3% of patients who were treated with recombinant activated FVII. Of the 163 patients who received immunosuppressive therapy, 80 (49.1%) received rituximab-based therapy with a 93.3% complete remission (CR) rate, 50 (30.7%) received steroids plus cyclophosphamide with an 85.0% CR rate, and 22 (13.5%) received steroids alone with an 82.4% CR rate. Six cases relapsed after a median duration of 330 days. Immunosuppressive therapy-related adverse events were reported in 17 patients. Seven deaths were recorded. FVIII inhibitor titer of ≥15 BU/mL and BS of ≥6 were identified as significantly poor prognostic factors for CR.
Conclusion: Immunosuppressive therapies yield remarkably high response rates, with a CR rate exceeding 80%; notably, the regimen containing rituximab exhibits a CR rate of approximately 90%. FVIII inhibitor titer of ≥5 BU/mL and BS of ≥6 were poor predictors of CR in patients with AHA.
Keywords: acquired hemophilia A; bleeding; immunosuppression; inhibitor; prognosis.
© 2024 Published by Elsevier Inc. on behalf of International Society on Thrombosis and Haemostasis.
Figures
Similar articles
-
Acquired Hemophilia A: A Retrospective Multicenter Analysis of 42 Patients.Clin Appl Thromb Hemost. 2023 Jan-Dec;29:10760296221151165. doi: 10.1177/10760296221151165. Clin Appl Thromb Hemost. 2023. PMID: 36653966 Free PMC article.
-
Inhibitor eradication and bleeding management of acquired hemophilia A: a single center experience in China.Hematology. 2019 Dec;24(1):631-636. doi: 10.1080/16078454.2019.1663028. Hematology. 2019. PMID: 31514689
-
[Clinical Analysis of 25 Cases of Acquired Hemophilia A in a Single Center].Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2024 Dec;32(6):1829-1833. doi: 10.19746/j.cnki.issn.1009-2137.2024.06.029. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2024. PMID: 39743272 Chinese.
-
Twelve years of experience of acquired hemophilia A: trials and tribulations in South Australia.Semin Thromb Hemost. 2009 Nov;35(8):769-77. doi: 10.1055/s-0029-1245109. Epub 2010 Feb 18. Semin Thromb Hemost. 2009. PMID: 20169513 Review.
-
Acquired Hemophilia A: An Update on the Etiopathogenesis, Diagnosis, and Treatment.Diagnostics (Basel). 2023 Jan 23;13(3):420. doi: 10.3390/diagnostics13030420. Diagnostics (Basel). 2023. PMID: 36766524 Free PMC article. Review.
Cited by
-
Laboratory Assessment of Factor VIII Inhibitors: When Is It Required? A Perspective Informed by Local Practice.J Clin Med. 2024 Dec 24;14(1):13. doi: 10.3390/jcm14010013. J Clin Med. 2024. PMID: 39797095 Free PMC article.
-
Emicizumab as first-line therapy in acquired hemophilia A.Res Pract Thromb Haemost. 2024 May 13;8(4):102438. doi: 10.1016/j.rpth.2024.102438. eCollection 2024 May. Res Pract Thromb Haemost. 2024. PMID: 38953052 Free PMC article.
-
Genetics and Epigenetics in Acquired Hemophilia A: From Bench to Bedside.Curr Issues Mol Biol. 2024 May 23;46(6):5147-5160. doi: 10.3390/cimb46060309. Curr Issues Mol Biol. 2024. PMID: 38920981 Free PMC article. Review.
References
-
- Collins P., Macartney N., Davies R., Lees S., Giddings J., Majer R. A population based, unselected, consecutive cohort of patients with acquired haemophilia A. Br J Haematol. 2004;124:86–90. - PubMed
-
- Tay L., Duncan E., Singhal D., Al-Qunfoidi R., Coghlan D., Jaksic W., et al. Twelve years of experience of acquired hemophilia A: trials and tribulations in South Australia. Semin Thromb Hemost. 2009;35:769–777. - PubMed
-
- Franchini M., Vaglio S., Marano G., Mengoli C., Gentili S., Pupella S., et al. Acquired hemophilia A: a review of recent data and new therapeutic options. Hematology. 2017;22:514–520. - PubMed
LinkOut - more resources
Full Text Sources

