Immunothrombosis versus thrombo-inflammation: platelets in cerebrovascular complications
- PMID: 38433977
- PMCID: PMC10907225
- DOI: 10.1016/j.rpth.2024.102344
Immunothrombosis versus thrombo-inflammation: platelets in cerebrovascular complications
Abstract
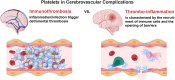
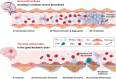
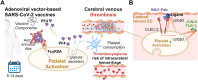
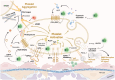
A State-of-the Art lecture titled "Thrombo-Neuroinflammatory Disease" was presented at the International Society on Thrombosis and Haemostasis Congress in 2023. First, we would like to advocate for discrimination between immunothrombosis and thrombo-inflammation, as immunothrombosis describes an overshooting inflammatory reaction that results in detrimental thrombotic activity. In contrast, thrombo-inflammation describes the interplay of platelets and coagulation with the immunovascular system, resulting in the recruitment of immune cells and loss of barrier function (hence, hallmarks of inflammation). Both processes can be observed in the brain, with cerebral venous thrombosis being a prime example of immunothrombosis, while infarct progression in response to ischemic stroke is a paradigmatic example of thrombo-inflammation. Here, we review the pathomechanisms underlying cerebral venous thrombosis and ischemic stroke from a platelet-centric perspective and discuss translational implications. Finally, we summarize relevant new data on this topic presented during the 2023 International Society on Thrombosis and Haemostasis Congress.
Keywords: blood platelets; cerebral venous thrombosis; immunothrombosis; ischemic stroke; thrombo-inflammation.
© 2024 The Author(s).
Figures
Similar articles
-
Immunothrombosis in neurovascular disease.Res Pract Thromb Haemost. 2023 Dec 20;8(1):102298. doi: 10.1016/j.rpth.2023.102298. eCollection 2024 Jan. Res Pract Thromb Haemost. 2023. PMID: 38292352 Free PMC article.
-
Platelet in thrombo-inflammation: Unraveling new therapeutic targets.Front Immunol. 2022 Nov 14;13:1039843. doi: 10.3389/fimmu.2022.1039843. eCollection 2022. Front Immunol. 2022. PMID: 36451834 Free PMC article. Review.
-
Novel mechanisms of thrombo-inflammation during infection: spotlight on neutrophil extracellular trap-mediated platelet activation.Res Pract Thromb Haemost. 2023 Mar 11;7(2):100116. doi: 10.1016/j.rpth.2023.100116. eCollection 2023 Feb. Res Pract Thromb Haemost. 2023. PMID: 37063765 Free PMC article.
-
Coagulation biomarkers for ischemic stroke.Res Pract Thromb Haemost. 2023 Apr 23;7(4):100160. doi: 10.1016/j.rpth.2023.100160. eCollection 2023 May. Res Pract Thromb Haemost. 2023. PMID: 37274178 Free PMC article.
-
Platelets in Thrombo-Inflammation: Concepts, Mechanisms, and Therapeutic Strategies for Ischemic Stroke.Hamostaseologie. 2020 Jun;40(2):153-164. doi: 10.1055/a-1151-9519. Epub 2020 May 26. Hamostaseologie. 2020. PMID: 32455457 Review.
Cited by
-
Targeting the P-selectin/PSGL-1 pathway: discovery of disease-modifying therapeutics for disorders of thromboinflammation.Blood Vessel Thromb Hemost. 2024 Jun 18;1(3):100015. doi: 10.1016/j.bvth.2024.100015. eCollection 2024 Sep. Blood Vessel Thromb Hemost. 2024. PMID: 40766814 Free PMC article. Review.
-
Treatment of Acute and Long-COVID, Diabetes, Myocardial Infarction, and Alzheimer's Disease: The Potential Role of a Novel Nano-Compound-The Transdermal Glutathione-Cyclodextrin Complex.Antioxidants (Basel). 2024 Sep 12;13(9):1106. doi: 10.3390/antiox13091106. Antioxidants (Basel). 2024. PMID: 39334765 Free PMC article. Review.
References
-
- Stoll G., Nieswandt B. Thrombo-inflammation in acute ischaemic stroke – implications for treatment. Nat Rev Neurol. 2019;15:473–481. - PubMed
-
- van der Meijden P.E.J., Heemskerk J.W.M. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16:166–179. - PubMed
-
- Uhrin P., Zaujec J., Breuss J.M., Olcaydu D., Chrenek P., Stockinger H., et al. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. - PubMed
LinkOut - more resources
Full Text Sources

