Overcoming Central β-Sheet #6 (Cβ6) ALK Mutation (L1256F), TP53 Mutations and Short Forms of EML4-ALK v3/b and v5a/b Splice Variants are the Unmet Need That a Re-Imagined 5th-Generation (5G) ALK TKI Must Deliver
- PMID: 38433979
- PMCID: PMC10908247
- DOI: 10.2147/LCTT.S446878
Overcoming Central β-Sheet #6 (Cβ6) ALK Mutation (L1256F), TP53 Mutations and Short Forms of EML4-ALK v3/b and v5a/b Splice Variants are the Unmet Need That a Re-Imagined 5th-Generation (5G) ALK TKI Must Deliver
Abstract
Despite the development and approval of seven anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKIs) spanning over three "generations" since the discovery of ALK fusion positive (ALK+) non-small cell lung cancer (NSCLC), there remains intrinsic and acquired resistances to these approved TKIs. Currently, a fourth-generation (4G) ALK TKI, NVL-655, is being developed to attack some of the unmet needs such as compound resistance mutations in cis. However, EML4-ALK variant 3 and TP53 mutations are intrinsic genomic alterations that negatively modulate efficacy of ALK TKIs. Potentially, in the shifting landscape where lorlatinib should be the first-line ALK TKI of choice based on the CROWN trial, the central β-sheet #6 (Cβ6) mutation ALK L1256F will be the potential acquired resistance mutation to lorlatinib which may be resistant to current ALK TKIs. Here we opine on what additional capacities a putative fifth-generation (5G) ALK TKI will need to possess if it can be achieved in one single molecule. We propose randomized trial schemas targeting some of the intrinsic resistance mechanisms that will lead to approval of a prototypic fifth-generation (5G) ALK TKI and actually be beneficial to ALK+ NSCLC patients rather than just design a positive pivotal superiority trial for the sole purpose of drug approval.
Keywords: ALK TKIs; ALK+ NSCLC; Cβ6 mutation; EML4-ALK variant 3a/b; TP53 mutation; circulating tumor DNA; fifth generation ALK TKI; next-generation sequencing.
© 2024 Lee and Ou.
Conflict of interest statement
Dr Sai-Hong Ignatius Ou reports honoraria from AnHeart Therapeutics, BMS, Claris Life Science, Pfizer, JNJ/Janssen, Daiichi Sankyo, Eli Lilly, OncLive, and DAVA Oncology LLP; has received research funding to his institution from BluePrint Medicines, Daiichi Sankyo, ERASCA Theper- atucis, Janssen/JNJ, Merus, Mirati Thepereutics, Merck, Nuvalent, Pfizer, Roche, Revolution Medicine, Sanofi, and Takeda; is a scientific advisory board member of AnHeart Therapeutics and Elevation Oncology; having stock ownership in Turning Point Therapeutics, Elevation Oncology, MBrace Therapeutics, BlossomHill Therapeutics, Lilly, Nuvalent, and Theseus Therapeutics, outside the submitted work. Dr. Lee reports no conflicts of interest in this work.
Figures
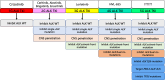


References
-
- Duruisseaux M, Besse B, Cadranel J, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget. 2017;8(13):21903–21917. doi:10.18632/oncotarget.15746 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

