Low-cost, open-source device for simultaneously subjecting rodents to different circadian cycles of light, food, and temperature
- PMID: 38434139
- PMCID: PMC10904513
- DOI: 10.3389/fphys.2024.1356787
Low-cost, open-source device for simultaneously subjecting rodents to different circadian cycles of light, food, and temperature
Abstract
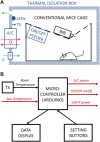
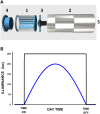
Exposure of experimental rodents to controlled cycles of light, food, and temperature is important when investigating alterations in circadian cycles that profoundly influence health and disease. However, applying such stimuli simultaneously is difficult in practice. We aimed to design, build, test, and open-source describe a simple device that subjects a conventional mouse cage to independent cycles of physiologically relevant environmental variables. The device is based on a box enclosing the rodent cage to modify the light, feeding, and temperature environments. The device provides temperature-controlled air conditioning (heating or cooling) by a Peltier module and includes programmable feeding and illumination. All functions are set by a user-friendly front panel for independent cycle programming. Bench testing with a model simulating the CO2 production of mice in the cage showed: a) suitable air renewal (by measuring actual ambient CO2), b) controlled realistic illumination at the mouse enclosure (measured by a photometer), c) stable temperature control, and d) correct cycling of light, feeding, and temperature. The cost of all the supplies (retail purchased by e-commerce) was <300 US$. Detailed technical information is open-source provided, allowing for any user to reliably reproduce or modify the device. This approach can considerably facilitate circadian research since using one of the described low-cost devices for any mouse group with a given light-food-temperature paradigm allows for all the experiments to be performed simultaneously, thereby requiring no changes in the light/temperature of a general-use laboratory.
Keywords: animal research; circadian alteration; experimental model; intermittent fasting; light cycle; open-source hardware; temperature cycle.
Copyright © 2024 Farré, Rodríguez-Lázaro, Otero, Gavara, Sunyer, Farré, Gozal and Almendros.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


Similar articles
-
An open-source device for measuring food intake and operant behavior in rodent home-cages.Elife. 2021 Mar 29;10:e66173. doi: 10.7554/eLife.66173. Elife. 2021. PMID: 33779547 Free PMC article.
-
OpenTCC: An open source low-cost temperature-control chamber.HardwareX. 2020 Feb 24;7:e00099. doi: 10.1016/j.ohx.2020.e00099. eCollection 2020 Apr. HardwareX. 2020. PMID: 35495215 Free PMC article.
-
Device for Negative Pressure Wound Therapy in Low-Resource Regions: Open-Source Description and Bench Test Evaluation.J Clin Med. 2022 Sep 15;11(18):5417. doi: 10.3390/jcm11185417. J Clin Med. 2022. PMID: 36143070 Free PMC article.
-
Safety and nutritional assessment of GM plants and derived food and feed: the role of animal feeding trials.Food Chem Toxicol. 2008 Mar;46 Suppl 1:S2-70. doi: 10.1016/j.fct.2008.02.008. Epub 2008 Feb 13. Food Chem Toxicol. 2008. PMID: 18328408 Review.
-
Open-Source Hardware May Address the Shortage in Medical Devices for Patients with Low-Income and Chronic Respiratory Diseases in Low-Resource Countries.J Pers Med. 2022 Sep 13;12(9):1498. doi: 10.3390/jpm12091498. J Pers Med. 2022. PMID: 36143283 Free PMC article. Review.
References
-
- American Society of Heating (2022). Refrigerating and air-conditioning engineers (ASHRAE). ASHRAE position document on indoor carbon dioxide. Approved by ASHRAE Board of Directors, February 2 Available at: https://www.ashrae.org/file%20library/about/position%20documents/pd_indo... .
LinkOut - more resources
Full Text Sources

