Mitotherapy inhibits against tenofovir induced nephrotoxicity on rat renal proximal tubular cells
- PMID: 38434141
- PMCID: PMC10907186
- DOI: 10.1016/j.bbrep.2024.101669
Mitotherapy inhibits against tenofovir induced nephrotoxicity on rat renal proximal tubular cells
Abstract
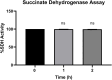
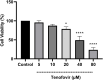
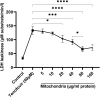
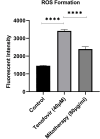
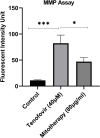
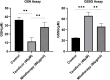
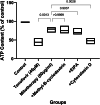
Tenofovir, as nucleotide reverse transcriptase inhibitors (NRTIs), is used to prevent and cure HIV/AIDS. Ample evidence confirmed that the nephrotoxicity of tenofovir has been linked to mitochondrial dysfunction. It seems that transplantation with healthy mitochondria instead of damaged mitochondria may be a beneficial approach to therapy. Therefore, it decided to investigate the impact of mitotherapy on tenofovir against renal proximal tubular cells (RPTCs) toxicity by measurement of oxidative stress and cytotoxicity biomarkers and restoring of mitochondrial function on isolated mitochondria. EC50 of tenofovir was achieved at 40 μM following 2 h incubation in Earle's solution (pH = 7.4; 37 °C). Freshly isolated mitochondria (80 μg/ml) were added to damage RPTCs affected by tenofovir in treated groups. One Way ANOVA analysis showed that healthy mitochondrial transplantation decreased oxidative stress biomarkers following tenofovir toxicity in RPTCs. Our data revealed that mitotherapy makes cell survival possible in RPTCs affected by tenofovir. In addition, it supposed that a novel and ideal strategy for the treatment of chemicals-induced nephrotoxicity.
Keywords: Mitochondrial transplantation; Nephrotoxicity; Oxidative stress; Renal proximal tubular cells (RPTCs); Tenofovir.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
The Effect of Donor Rat Gender in Mitochondrial Transplantation Therapy of Cisplatin-Induced Toxicity on Rat Renal Proximal Tubular Cells.Iran J Pharm Res. 2023 Apr 8;22(1):e135666. doi: 10.5812/ijpr-135666. eCollection 2023 Jan-Dec. Iran J Pharm Res. 2023. PMID: 38148888 Free PMC article.
-
Mitochondrial Transplantation Therapy against Ifosfamide Induced Toxicity on Rat Renal Proximal Tubular Cells.Drug Res (Stuttg). 2023 Feb;73(2):113-120. doi: 10.1055/a-1967-2066. Epub 2022 Nov 17. Drug Res (Stuttg). 2023. PMID: 36395822
-
Mitochondrial transplantation against gentamicin-induced toxicity on rat renal proximal tubular cells: the higher activity of female rat mitochondria.In Vitro Cell Dev Biol Anim. 2023 Jan;59(1):31-40. doi: 10.1007/s11626-022-00743-1. Epub 2023 Jan 11. In Vitro Cell Dev Biol Anim. 2023. PMID: 36630058
-
Tenofovir-induced nephrotoxicity: incidence, mechanism, risk factors, prognosis and proposed agents for prevention.Eur J Clin Pharmacol. 2014 Sep;70(9):1029-40. doi: 10.1007/s00228-014-1712-z. Epub 2014 Jun 25. Eur J Clin Pharmacol. 2014. PMID: 24958564 Review.
-
Proximal tubular renal dysfunction or damage in HIV-infected patients.AIDS Rev. 2012 Jul-Sep;14(3):179-87. AIDS Rev. 2012. PMID: 22833061 Review.
Cited by
-
Direct exposure with exogenous mitochondria reduce colistin-induced mitochondrial dysfunction and cellular damages in isolated rat renal proximal tubular cells.J Mol Histol. 2025 Mar 22;56(2):114. doi: 10.1007/s10735-025-10389-4. J Mol Histol. 2025. PMID: 40119251
References
-
- De Clercq E. In search of a selective therapy of viral infections. Antivir. Res. 2010;85(1):19–24. - PubMed
-
- Dietz J.-P., et al. Di-tert-butyl phosphonate route to the antiviral drug tenofovir. Org. Process Res. Dev. 2021;25(4):789–798. - PubMed
-
- Zimmermann A.E., et al. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin. Infect. Dis. 2006;42(2):283–290. - PubMed
-
- Lewis W., Copeland W.C., Day B.J. Mitochondrial DNA depletion, oxidative stress, and mutation: mechanisms 0f dysfunction from nucleoside reverse transcriptase inhibitors. Lab. Invest. 2001;81(6):777–790. - PubMed
-
- Lewis W. Nucleoside reverse transcriptase inhibitors, mitochondrial DNA and AIDS therapy. Antivir. Ther. 2005;10(2_suppl):13–27. - PubMed
LinkOut - more resources
Full Text Sources

