Cellular and Molecular Mechanisms of Toxic Liver Fibrosis in Rats Depending on the Stages of Its Development
- PMID: 38434195
- PMCID: PMC10902903
- DOI: 10.17691/stm2023.15.4.05
Cellular and Molecular Mechanisms of Toxic Liver Fibrosis in Rats Depending on the Stages of Its Development
Abstract
The aim is to study the cellular and molecular features of toxic liver fibrosis in rats and its dependence on development stages of this pathological condition.
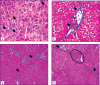
Materials and methods: Liver fibrogenesis in male Wistar rats was induced with the thioacetamide solution by introducing into the stomach with a probe at a dose of 200 mg/kg of animal body weight 2 times per week. The process dynamics was studied at 5 time points (control, week 3, week 5, week 7, and week 9). The mRNA levels of tweak, fn14, ang, vegfa, cxcl12, and mmp-9 genes in liver were detected by real-time polymerase chain reaction. Immunohistochemical study was performed on paraffin sections. The CD31, CD34, CK19, α-SMA, FAP, CD68, CD206, CX3CR1, and CD45 cells were used as markers. Fibrosis degree was determined in histological sections, stained in line with the Mallory technique, according to the Ishak's semi-quantitative scale.
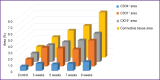
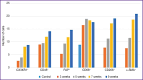
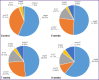
Results: Two simultaneously existing morphologically heterogeneous populations of myofibroblasts expressing different types of markers (FAP, α-SMA) were identified in rat liver. Prior to the onset of transformation of fibrosis into cirrhosis (F1-F4, weeks 3-7), FAP+ and SMA+ cells were localized in different places on histological specimens. All stages of liver fibrosis development were accompanied by an increase in the number (p=0.0000), a change in the phenotypic structure and functional properties of macrophages. The CK19+ cells of the portal areas differentiated into cholangiocytes that formed interlobular bile ducts and ductules, as well as hepatocytes that formed rudiments of new hepatic microlobules. Pathological venous angiogenesis and heterogeneity of endotheliocytes of the intrahepatic vascular bed were detected. Two options for changes in mRNA expression of the selected genes were identified. The level of the fn14 and mmp-9 mRNAs at all stages of fibrosis was higher (p=0.0000) than in control rats. For tweak, ang, vegfa, and cxcl12 mRNAs, the situation was the opposite - the level of genes decreased (p=0.0000). There were strong and moderate correlations between the studied target genes (p<0.05).
Conclusion: It was established that the stages of toxic fibrosis had morphological and molecular genetic features. The FAP+ cells make the main contribution to development of portal and initial stage of bridging fibrosis. The stellate macrophages and infiltrating monocytes/ macrophages can potentially be used for development of new therapeutic strategies for liver pathology treatment. One should take into account the features of the markers' expression by endothelial cells during the study of the intrahepatic vascular bed. Joint study of genes is a necessary ad-hoc parameter in fundamental and preclinical research.
Keywords: Ishak’s scale; immunohistochemistry; mRNA expression; rat liver fibrogenesis; toxic fibrosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
[Relationships between Cxcl12, Tweak, Notch1, and Yap mRNA Expression Levels in Molecular Mechanisms of Liver Fibrogenesis].Mol Biol (Mosk). 2024 Jan-Feb;58(1):130-140. Mol Biol (Mosk). 2024. PMID: 38943584 Russian.
-
literammp-9 mRNA Expression and Bridging Fibrosis Progression in Toxic Liver Injury.Acta Naturae. 2023 Apr-Jun;15(2):50-58. doi: 10.32607/actanaturae.17856. Acta Naturae. 2023. PMID: 37538808 Free PMC article.
-
Changes in Macrophage Subpopulations in Rat Liver at Different Stages of Experimental Fibrosis.Bull Exp Biol Med. 2023 Jun;175(2):279-285. doi: 10.1007/s10517-023-05850-x. Epub 2023 Jul 21. Bull Exp Biol Med. 2023. PMID: 37477742
-
[Inhibitory effect of acupuncture on hepatic extracellular matrix production in carbon tetrachloride-induced liver fibrosis rats].Zhen Ci Yan Jiu. 2012 Feb;37(1):8-14. Zhen Ci Yan Jiu. 2012. PMID: 22574562 Chinese.
-
The immunology of fibrogenesis in alcoholic liver disease.Arch Pathol Lab Med. 2004 Nov;128(11):1230-8. doi: 10.5858/2004-128-1230-TIOFIA. Arch Pathol Lab Med. 2004. PMID: 15504057
Cited by
-
The Role of Activated Stromal Cells in Fibrotic Foci Formation and Reversion.Cells. 2024 Dec 13;13(24):2064. doi: 10.3390/cells13242064. Cells. 2024. PMID: 39768155 Free PMC article.
References
-
- Xu M., Xu H.H., Lin Y., Sun X., Wang L.J., Fang Z.P., Su X.H., Liang X.J., Hu Y., Liu Z.M., Cheng Y., Wei Y., Li J., Li L., Liu H.J., Cheng Z., Tang N., Peng C., Li T., Liu T., Qiao L., Wu D., Ding Y.Q., Zhou W.J. LECT2, a ligand for Tie1, plays a crucial role in liver fibrogenesis. Cell. 2019;178(6):1478–1492.e20. doi: 10.1016/j.cell.2019.07.021. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
