Cortical neurons obtained from patient-derived iPSCs with GNAO1 p.G203R variant show altered differentiation and functional properties
- PMID: 38434323
- PMCID: PMC10907651
- DOI: 10.1016/j.heliyon.2024.e26656
Cortical neurons obtained from patient-derived iPSCs with GNAO1 p.G203R variant show altered differentiation and functional properties
Abstract
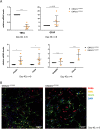
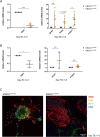
Pathogenic variants in the GNAO1 gene, encoding the alpha subunit of an inhibitory heterotrimeric guanine nucleotide-binding protein (Go) highly expressed in the mammalian brain, have been linked to encephalopathy characterized by different combinations of neurological symptoms, including developmental delay, hypotonia, epilepsy and hyperkinetic movement disorder with life-threatening paroxysmal exacerbations. Currently, there are only symptomatic treatments, and little is known about the pathophysiology of GNAO1-related disorders. Here, we report the characterization of a new in vitro model system based on patient-derived induced pluripotent stem cells (hiPSCs) carrying the recurrent p.G203R amino acid substitution in Gαo, and a CRISPR-Cas9-genetically corrected isogenic control line. RNA-Seq analysis highlighted aberrant cell fate commitment in neuronal progenitor cells carrying the p.G203R pathogenic variant. Upon differentiation into cortical neurons, patients' cells showed reduced expression of early neural genes and increased expression of astrocyte markers, as well as premature and defective differentiation processes leading to aberrant formation of neuronal rosettes. Of note, comparable defects in gene expression and in the morphology of neural rosettes were observed in hiPSCs from an unrelated individual harboring the same GNAO1 variant. Functional characterization showed lower basal intracellular free calcium concentration ([Ca2+]i), reduced frequency of spontaneous activity, and a smaller response to several neurotransmitters in 40- and 50-days differentiated p.G203R neurons compared to control cells. These findings suggest that the GNAO1 pathogenic variant causes a neurodevelopmental phenotype characterized by aberrant differentiation of both neuronal and glial populations leading to a significant alteration of neuronal communication and signal transduction.
Keywords: Encephalopathy; GNAO1; Induced pluripotent stem cell; Movement disorder; neural rosette; wnt.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Gnao1 is a molecular switch that regulates the Rho signaling pathway in differentiating neurons.Sci Rep. 2024 Jul 24;14(1):17097. doi: 10.1038/s41598-024-68062-x. Sci Rep. 2024. PMID: 39048611 Free PMC article.
-
Mouse models characterize GNAO1 encephalopathy as a neurodevelopmental disorder leading to motor anomalies: from a severe G203R to a milder C215Y mutation.Acta Neuropathol Commun. 2022 Jan 28;10(1):9. doi: 10.1186/s40478-022-01312-z. Acta Neuropathol Commun. 2022. PMID: 35090564 Free PMC article.
-
Clinical and Molecular Profiling in GNAO1 Permits Phenotype-Genotype Correlation.Mov Disord. 2024 Sep;39(9):1578-1591. doi: 10.1002/mds.29881. Epub 2024 Jun 16. Mov Disord. 2024. PMID: 38881224
-
A mechanistic review on GNAO1-associated movement disorder.Neurobiol Dis. 2018 Aug;116:131-141. doi: 10.1016/j.nbd.2018.05.005. Epub 2018 May 24. Neurobiol Dis. 2018. PMID: 29758257 Review.
-
Phenotypic Diversity in GNAO1 Patients: A Comprehensive Overview of Variants and Phenotypes.Hum Mutat. 2023 Aug 7;2023:6628283. doi: 10.1155/2023/6628283. eCollection 2023. Hum Mutat. 2023. PMID: 40225165 Free PMC article. Review.
Cited by
-
Gnao1 is a molecular switch that regulates the Rho signaling pathway in differentiating neurons.Sci Rep. 2024 Jul 24;14(1):17097. doi: 10.1038/s41598-024-68062-x. Sci Rep. 2024. PMID: 39048611 Free PMC article.
-
Developmental and Epileptic Encephalopathy: Pathogenesis of Intellectual Disability Beyond Channelopathies.Biomolecules. 2025 Jan 15;15(1):133. doi: 10.3390/biom15010133. Biomolecules. 2025. PMID: 39858526 Free PMC article. Review.
-
A Personalized 14-3-3 Disease-Targeting Workflow Yields Repositioning Drug Candidates.Cells. 2025 Apr 8;14(8):559. doi: 10.3390/cells14080559. Cells. 2025. PMID: 40277885 Free PMC article.
-
Personalized allele-specific antisense oligonucleotides for GNAO1-neurodevelopmental disorder.Mol Ther Nucleic Acids. 2024 Dec 22;36(1):102432. doi: 10.1016/j.omtn.2024.102432. eCollection 2025 Mar 11. Mol Ther Nucleic Acids. 2024. PMID: 39897576 Free PMC article.
-
Human iPSC-Derived Neuron and Oligodendrocyte Co-culture as a Small-Molecule Screening Assay for Myelination.Bio Protoc. 2025 May 5;15(9):e5227. doi: 10.21769/BioProtoc.5227. eCollection 2025 May 5. Bio Protoc. 2025. PMID: 40364981 Free PMC article.
References
-
- Nakamura K., Kodera H., Akita T., Shiina M., Kato M., Hoshino H., Terashima H., Osaka H., Nakamura S., Tohyama J., Kumada T. De novo mutations in GNAO1, encoding a Gαo subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am. J. Hum. Genet. 2013;93(3):496–505. doi: 10.1016/j.ajhg.2013.07.014. - DOI - PMC - PubMed
-
- Ananth A.L., Robichaux-Viehoever A., Kim Y.M., Hanson-Kahn A., Cox R., Enns G.M., Strober J., Willing M., Schlaggar B.L., Wu Y.W., Bernstein J.A. Clinical course of six children with GNAO1 mutations causing a severe and distinctive movement disorder. Pediatr. Neurol. 2016;59:81–84. doi: 10.1016/j.pediatrneurol.2016.02.018. - DOI - PubMed
-
- Galosi S., Novelli M., Di Rocco M., Flex E., Messina E., Pollini L., Parrini E., Pisani F., Guerrini R., Leuzzi V., Martinelli S. GNAO1 haploinsufficiency: the milder end of the GNAO1 phenotypic spectrum. Movement disorders. official journal of the Movement Disorder Society. 2023 doi: 10.1002/mds.29585. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

