Interfacial Tuning of Electrocatalytic Ag Surfaces for Fragment-Based Electrophile Coupling
- PMID: 38434422
- PMCID: PMC10906991
- DOI: 10.1038/s41929-023-01073-5
Interfacial Tuning of Electrocatalytic Ag Surfaces for Fragment-Based Electrophile Coupling
Abstract
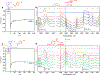
Construction of C‒C bonds in medicinal chemistry frequently draws on the reductive coupling of organic halides with ketones or aldehydes. Catalytic C(sp3)‒C(sp3) bond formation, however, is constrained by the competitive side reactivity of radical intermediates following sp3 organic halide activation. Here, an alternative paradigm deploys catalytic Ag surfaces for reductive fragment-based electrophile coupling compatible with sp3 organic halides. We use in-situ spectroscopy, electrochemical analyses, and simulation to uncover the catalytic interfacial structure and guide reaction development. Specifically, Mg(OAc)2 outcompetes the interaction between Ag and the aldehyde, thereby tuning the Ag surface for selective product formation. Data are consistent with an increased population of Mg-bound aldehyde facilitating the addition of a carbon-centered radical (product of Ag-electrocatalyzed organic halide reduction) to the carbonyl. Electron transfer from Ag to the resultant alkoxy radical yields the desired alcohol. Molecular interfacial tuning at reusable catalytic electrodes will accelerate development of sustainable organic synthetic methods.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures

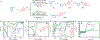

References
-
- Lovering F, Bikker J & Humblet C Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem 52, 6752–6756 (2009). - PubMed
-
- Huffman BJ et al. Electronic complementarity permits hindered butenolide heterodimerization and discovery of novel cGAS/STING pathway antagonists. Nat. Chem 12, 310–317 (2020). - PubMed
-
- Wei W, Cherukupalli S, Jing L, Liu X & Zhan P Fsp3: a new parameter for drug-likeness. Drug Discov. Today 25, 1839–1845 (2020). - PubMed
-
- Rappoport Z & Marek I The Chemistry of Organomagnesium Compounds. (John Wiley & Sons, Ltd, 2008).
-
- Wu G & Huang M Organolithium reagents in pharmaceutical asymmetric processes. Chem. Rev 106, 2596–2616 (2006). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
