Evaluation of medication safety assessment tools for pharmacist-led medication reviews: the Eastern European pilot project
- PMID: 38434703
- PMCID: PMC10904472
- DOI: 10.3389/fphar.2024.1348400
Evaluation of medication safety assessment tools for pharmacist-led medication reviews: the Eastern European pilot project
Abstract
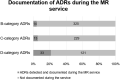
Background: Pharmacist-led medication reviews (MR) are one of the key methods to support medication safety in polypharmacy patients. The aims of this study were to pilot MRs in Eastern European community pharmacies, describe medication use in polypharmacy patients, and evaluate the usability of medication safety assessment tools. Methods: The MR pilot was undertaken in Estonia, Latvia, Poland, Hungary, Romania, and Bulgaria. Patients who used at least five medicines were directed to the service by their GPs. Data on drug-related problems (DRPs) and adherence were collected by pharmacists through structured patient interviews. Databases for identification of potential drug-drug interactions (pDDIs) and adverse drug reactions (ADRs) named Inxbase/Riskbase, as well as an integrated tool comprising potentially inappropriate medicines (PIMs) lists EU(7)-PIM and EURO-FORTA, were applied retroactively to the MR pilot data to investigate possibilities for their use and to describe medication use and potential risks in the study population. Results: A total of 318 patients were included in the study, 250 of them elderly (≥65 years). One hundred and eighty (56.6%) participants had a total of 504 pDDIs based on Inxbase analysis. On average, there were 1.6 pDDIs per participant. Twenty-five (5.0%) of the 504 pDDIs were in a high-risk category. A total of 279 (87.7%) participants had a potential ADR in at least one of 10 Riskbase categories. One hundred and fifty-four (20.8%) of the potential ADRs were in a high-risk category. Twenty-seven pDDIs and 68 ADRs documented as DRPs during the service were not included in the databases. Using the integrated EU(7)-PIM/EURO-FORTA PIM list, a total of 816 PIMs were found in 240 (96%) of the 250 elderly participants (on average 3.4 PIMs per elderly participant). Seventy-one (29.6%) of the participants were using high-risk PIMs. Twenty-one percent of high-risk PIMs and 13.8% of medium-risk PIMs were documented as DRPs by the pharmacists during the pilot. Conclusion: Medication safety assessment tools can be useful in guiding decision-making during MRs; however, these tools cannot replace patient interviews and monitoring. Tools that include a thorough explanation of the potential risks and are easy to use are more beneficial for MRs.
Keywords: Eastern Europe; PIM list; adverse drug events; community pharmacy services; medication use review; polypharmacy.
Copyright © 2024 Tuula, Merks, Waszyk-Nowaczyk, Drozd, Petrova, Viola, Bobrova, Scott, Oona and Volmer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bechman K., Clarke B. D., Rutherford A. I., Yates M., Nikiphorou E., Molokhia M., et al. (2019). Polypharmacy is associated with treatment response and serious adverse events: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology 58 (10), 1767–1776. 10.1093/rheumatology/kez037 - DOI - PubMed
-
- Brkic J., Fialova D., Okuyan B., Kummer I., Sesto S., Capiau A., et al. (2022). Prevalence of potentially inappropriate prescribing in older adults in Central and Eastern Europe: a systematic review and synthesis without meta-analysis. Sci. Rep. 12 (1), 16774. 10.1038/s41598-022-19860-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

