Adverse drug events associated with linezolid administration: a real-world pharmacovigilance study from 2004 to 2023 using the FAERS database
- PMID: 38434706
- PMCID: PMC10904462
- DOI: 10.3389/fphar.2024.1338902
Adverse drug events associated with linezolid administration: a real-world pharmacovigilance study from 2004 to 2023 using the FAERS database
Abstract
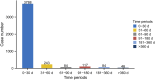
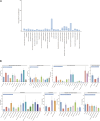
Introduction: Linezolid is an oxazolidinone antibiotic that is active against drug-resistant Gram-positive bacteria and multidrug-resistant Mycobacterium tuberculosis. Real-world studies on the safety of linezolid in large populations are lacking. This study aimed to determine the adverse events associated with linezolid in real-world settings by analyzing data from the US Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS). Methods: We retrospectively extracted reports on adverse drug events (ADEs) from the FAERS database from the first quarter of 2004 to that of 2023. By using disproportionality analysis including reporting odds ratio (ROR), proportional reporting ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), along with the multi-item gamma Poisson shrinker (MGPS), we evaluated whether there was a significant association between linezolid and ADE. The time to onset of ADE was further analyzed in the general population and within each age, weight, reporting population, and weight subgroups. Results: A total of 11,176 reports of linezolid as the "primary suspected" drug and 263 significant adverse events of linezolid were identified, including some common adverse events such as thrombocytopenia (n = 1,139, ROR 21.98), anaemia (n = 704, ROR 7.39), and unexpected signals that were not listed on the drug label such as rhabdomyolysis (n = 90, ROR 4.33), and electrocardiogram QT prolonged (n = 73, ROR 4.07). Linezolid-induced adverse reactions involved 27 System Organ Class (SOC). Gender differences existed in ADE signals related to linezolid. The median onset time of all ADEs was 6 days, and most ADEs (n = 3,778) occurred within the first month of linezolid use but some may continue to occur even after a year of treatment (n = 46). Conclusion: This study reports the time to onset of adverse effects in detail at the levels of SOC and specific preferred term (PT). The results of our study provide valuable insights for optimizing the use of linezolid and reducing potential side effects, expected to facilitate the safe use of linezolid in clinical settings.
Keywords: FAERS; adverse drug event; disproportionality analysis; linezolid; pharmacovigilance; real-world analysis.
Copyright © 2024 Zou, Cui, Lou, Ou, Zhu, Shu, Chen, Zhao, Wu, Wang, Chen, Chen and Lan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Safety evaluation of irinotecan: a real-world disproportionality analysis using FAERS and JADER databases during the time period 2004-2024.Front Pharmacol. 2025 Jun 9;16:1516449. doi: 10.3389/fphar.2025.1516449. eCollection 2025. Front Pharmacol. 2025. PMID: 40552159 Free PMC article.
-
Post-marketing safety profile of ganirelix in women: a 20-year pharmacovigilance analysis of global adverse drug event databases (2004-2024).BMC Pharmacol Toxicol. 2025 Apr 22;26(1):91. doi: 10.1186/s40360-025-00920-4. BMC Pharmacol Toxicol. 2025. PMID: 40264185 Free PMC article.
-
A multidimensional assessment of adverse events associated with paliperidone palmitate: a real-world pharmacovigilance study using the FAERS and JADER databases.BMC Psychiatry. 2025 Jan 20;25(1):52. doi: 10.1186/s12888-025-06493-0. BMC Psychiatry. 2025. PMID: 39833706 Free PMC article.
-
Post-marketing safety concerns with pirfenidone and nintedanib: an analysis of individual case safety reports from the FDA adverse event reporting system database and the Japanese adverse drug event report databases.Front Pharmacol. 2025 Apr 28;16:1530697. doi: 10.3389/fphar.2025.1530697. eCollection 2025. Front Pharmacol. 2025. PMID: 40356972 Free PMC article.
-
Guillain-Barré syndrome and checkpoint inhibitor therapy: insights from pharmacovigilance data.BMJ Neurol Open. 2024 Mar 13;6(1):e000544. doi: 10.1136/bmjno-2023-000544. eCollection 2024. BMJ Neurol Open. 2024. PMID: 38501128 Free PMC article. Review.
Cited by
-
Signal mining and analysis of trifluridine/tipiracil adverse events based on real-world data from the FAERS database.Front Pharmacol. 2024 Jul 23;15:1399998. doi: 10.3389/fphar.2024.1399998. eCollection 2024. Front Pharmacol. 2024. PMID: 39108741 Free PMC article.
-
Adverse drug events associated with insulin glargine: a real-world pharmacovigilance study based on the FAERS database.Front Pharmacol. 2025 Apr 28;16:1563238. doi: 10.3389/fphar.2025.1563238. eCollection 2025. Front Pharmacol. 2025. PMID: 40356973 Free PMC article.
-
High-risk adverse events in two types of single inhaler triple-therapy: a pharmacovigilance study based on the FAERS database.Front Pharmacol. 2025 Jan 9;15:1460407. doi: 10.3389/fphar.2024.1460407. eCollection 2024. Front Pharmacol. 2025. PMID: 39850558 Free PMC article.
-
A disproportionality analysis of adverse events associated with loop diuretics in the FDA Adverse Event Reporting System (FAERS).BMC Pharmacol Toxicol. 2025 Mar 17;26(1):63. doi: 10.1186/s40360-025-00890-7. BMC Pharmacol Toxicol. 2025. PMID: 40098168 Free PMC article.
-
Pharmacokinetics, tolerability, and safety of TBI-223, a novel oxazolidinone, in healthy participants.Antimicrob Agents Chemother. 2025 Apr 2;69(4):e0154224. doi: 10.1128/aac.01542-24. Epub 2025 Mar 11. Antimicrob Agents Chemother. 2025. PMID: 40067046 Free PMC article. Clinical Trial.
References
LinkOut - more resources
Full Text Sources

