Clinical trials and their impact on policy during COVID-19: a review
- PMID: 38434720
- PMCID: PMC10905118
- DOI: 10.12688/wellcomeopenres.19305.1
Clinical trials and their impact on policy during COVID-19: a review
Abstract
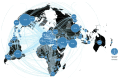
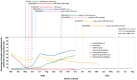
Background: Of over 8,000 recorded randomised trials addressing COVID-19, around 80% were of treatments, and 17% have reported results. Approximately 1% were adaptive or platform trials, with 25 having results available, across 29 journal articles and 10 preprint articles.
Methods: We conducted an extensive literature review to address four questions about COVID-19 trials, particularly the role and impact of platform/adaptive trials and lessons learned.
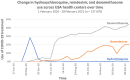
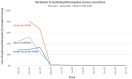
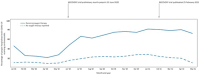
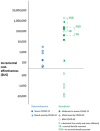
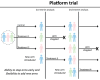
Results: The key findings were: Q1. Social value in conducting trials and uptake into policy? COVID-19 drug treatments varied substantially and changed considerably, with drugs found effective in definitive clinical trials replacing unproven drugs. Dexamethasone has likely saved ½-2 million lives, and was cost effective across a range of countries and populations, whereas the cost effectiveness of remdesivir is uncertain. Published economic and health system impacts of COVID-19 treatments were infrequent. Q2. Issues with adaptive trial designs. Of the 77 platform trials registered, 6 major platform trials, with approximately 50 treatment arms, recruited ~135,000 participants with funding over $100 million. Q3. Models of good practice. Streamlined set-up processes such as flexible and fast-track funding, ethics, and governance approvals are vital. To facilitate recruitment, simple and streamlined research processes, and pre-existing research networks to coordinate trial planning, design, conduct and practice change are crucial to success. Q4. Potential conflicts to avoid? When treating patients through trials, balancing individual and collective rights and allocating scarce resources between healthcare and research are challenging. Tensions occur between commercial and non-commercial sectors, and academic and public health interests, such as publication and funding driven indicators and the public good.
Conclusion: There is a need to (i) reduce small, repetitive, single centre trials, (ii) increase coordination to ensure robust research conducted for treatments, and (iii) a wider adoption of adaptive/platform trial designs to respond to fast-evolving evidence landscape.
Keywords: COVID-19; SARS-CoV-2; adaptive trials; clinical trials; health policy; pandemic; platform trials; treatment.
Copyright: © 2024 Glasziou P et al.
Conflict of interest statement
No competing interests were disclosed.
Figures




References
-
- Cochrane Community: About COVID-19 Study Register. 2022. Reference Source
-
- Epistemonikos Foundation: Methods and reports of COVID-19 L*OVE. 2022. Reference Source
-
- He Z, Erdengasileng A, Luo X, et al. : How the clinical research community responded to the COVID-19 pandemic: an analysis of the COVID-19 clinical studies in ClinicalTrials.gov. JAMIA Open. 2021;4(2): ooab032. 10.1093/jamiaopen/ooab032 - DOI - PMC - PubMed
-
- Sacks CA, North CM, Wolf M, et al. : The Landscape of COVID-19 Research in the United States: a Cross-sectional Study of Randomized Trials Registered on ClinicalTrials.Gov. J Gen Intern Med. 2022;37(1):154–61. 10.1007/s11606-021-07167-9 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

