Health Services Access Inequalities in Brazil Result in Poorer Outcomes for Stage III NSCLC-RELANCE/LACOG 0118
- PMID: 38434771
- PMCID: PMC10906523
- DOI: 10.1016/j.jtocrr.2024.100646
Health Services Access Inequalities in Brazil Result in Poorer Outcomes for Stage III NSCLC-RELANCE/LACOG 0118
Abstract
Introduction: Stage III NSCLC is a heterogeneous disease, representing approximately one-third of newly diagnosed lung cancers. Brazil lacks detailed information regarding stage distribution, treatment patterns, survival, and prognostic variables in locally advanced NSCLC.
Methods: RELANCE/LACOG 0118 is an observational, retrospective cohort study assessing sociodemographic and clinical data of patients diagnosed with having stage III NSCLC from January 2015 to June 2019, regardless of treatment received. The study was conducted across 13 cancer centers in Brazil. Disease status and survival data were collected up to June 2021. Descriptive statistics, survival analyses, and a multivariable Cox regression model were performed. p values less than 0.05 were considered significant.
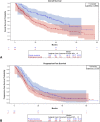
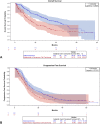
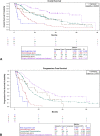
Results: We recruited 403 patients with stage III NSCLC. Most were male (64.0%), White (31.5%), and smokers or former smokers (86.1%). Most patients had public health insurance (67.5%), had stage IIIA disease (63.2%), and were treated with concurrent chemoradiation (53.1%). The median follow-up time was 33.83 months (95% confidence interval [CI]: 30.43-37.50). Median overall survival (OS) was 27.97 months (95% CI: 21.57-31.73), and median progression-free survival was 11.23 months (95% CI: 10.70-12.77). The type of treatment was independently associated with OS and progression-free survival, whereas the types of health insurance and histology were independent predictors of OS only.
Conclusions: Brazilian patients with stage III NSCLC with public health insurance are diagnosed later and have poorer OS. Nevertheless, patients with access to adequate treatment have outcomes similar to those reported in the pivotal trials. Health policy should be improved to make lung cancer diagnosis faster and guarantee prompt access to adequate treatment in Brazil.
Keywords: Epidemiology; Health insurance; NSCLC; Stage III; Survival.
© 2024 The Authors.
Conflict of interest statement
Dr. Cordeiro de Lima reports receiving research grant from 10.13039/100002491Bristol-Myers Squibb (for the institution); payment or honoraria from AstraZeneca, Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck Serono, and Novartis; support for attending meetings from 10.13039/100004325AstraZeneca and 10.13039/100004337Roche; and having participation on data safety monitoring board or advisory board in AstraZeneca, Daiichi Sankyo, Amgen, Pfizer, Eli Lilly, Merck Sharp & Dohme, Bristol-Myers Squibb, Merck Serono, and Janssen. Dr. De Castro Jr., reports having consulting or advisory roles from Boehringer Ingelheim, Pfizer, Bayer, Roche, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, Yuhan, Merck Serono, Janssen, Libbs, Sanofi, Novartis, Eli Lilly, Amgen, and Takeda; receiving payment or honoraria from AstraZeneca, Pfizer, Merck Sharp & Dohme, Bristol-Myers Squibb, Novartis, Roche, Amgen, Janssen, Merck Serono, Bayer, and Takeda; receiving support for attending meetings from Boehringer Ingelheim, 10.13039/100004319Pfizer, 10.13039/100004326Bayer, Roche, 10.13039/100009947Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, Merck Serono, 10.13039/100004336Novartis, Eli Lilly, and 10.13039/100002429Amgen; and having participation on data safety monitoring board or advisory board in Boehringer Ingelheim, Pfizer, Bayer, Roche, Merck Sharp & Dohme, Bristol-Myers Squibb, AstraZeneca, Yuhan, Merck Serono, Janssen, Libbs, Sanofi, Novartis, Eli Lilly, Amgen, and Takeda. Dr. Werutsky reports receiving grants or contracts from Novartis, Roche/10.13039/100004328Genentech, AstraZeneca/10.13039/501100004628MedImmune, Eli Lilly, 10.13039/100004330GlaxoSmithKline, Novartis, Pfizer, Bristol-Myers Squibb Brazil, Merck Sharp & Dohme, Merck, Bayer, 10.13039/100005565Janssen, Bristol-Myers Squibb, Astellas, Libbs, Takeda, 10.13039/100006436Celgene, and GlaxoSmithKline; consulting fees from Merck; and payment or honoraria for lectures from Pfizer, AstraZeneca/MedImmune, Libbs, and Merck. The remaining authors declare no conflict of interest.
Figures

References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Instituto Nacional de Câncer José Alencar Gomes Estimativa 2023: incidência de câncer no brasil. https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//...
-
- Howlader N., Noone A.M., Krapcho M., et al. SEER cancer statistics 1975–2014. National Cancer Institute. https://seer.cancer.gov/csr/1975_2014/
-
- Younes R.N., Deutsch F., Badra C., Gross J., Haddad F., Deheinzelin D. Non-small cell lung cancer: evaluation of 737 consecutive patients in a single institution. Rev Hosp Clin. 2004;59:119–127. - PubMed
-
- Mascarenhas E., Lessa G. Perfil clínico e sócio-demográfico de pacientes com câncer de pulmão não-pequenas células atendidos num serviço privado. Revista Brasileira de Oncologia Clínica. 2010;7:49–54.
LinkOut - more resources
Full Text Sources

