Synthesis and Trace-Level Quantification of Mutagenic and Cohort-of-Concern Ciprofloxacin Nitroso Drug Substance-Related Impurities (NDSRIs) and Other Nitroso Impurities Using UPLC-ESI-MS/MS-Method Optimization Using I-Optimal Mixture Design
- PMID: 38434810
- PMCID: PMC10905725
- DOI: 10.1021/acsomega.3c05170
Synthesis and Trace-Level Quantification of Mutagenic and Cohort-of-Concern Ciprofloxacin Nitroso Drug Substance-Related Impurities (NDSRIs) and Other Nitroso Impurities Using UPLC-ESI-MS/MS-Method Optimization Using I-Optimal Mixture Design
Abstract
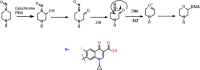
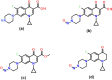
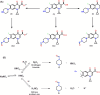
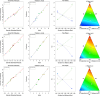
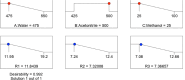
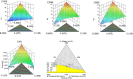
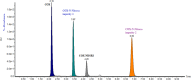
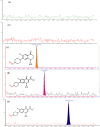
Globally, the pharmaceutical industry has been facing challenges from nitroso drug substance-related impurities (NDSRIs). In the current study, we synthesized and developed a rapid new UPLC-MS/MS method for the trace-level quantification of ciprofloxacin NDSRIs and a couple of N-nitroso impurities simultaneously. (Q)-SAR methodology was employed to assess and categorize the genotoxicity of all ciprofloxacin N-nitroso impurities. The projected results were positive, and the cohort of concern (CoC) for all three N-nitroso impurities indicates potential genotoxicity. AQbD-driven I-optimal mixture design was used to optimize the mixture of solvents in the method. The chromatographic resolution was accomplished using an Agilent Poroshell 120 Aq-C18 column (150 mm × 4.6 mm, 2.7 μm) in isocratic elution mode with 0.1% formic acid in a mixture of water, acetonitrile, and methanol in the ratio of 475:500:25 v/v/v at a flow rate of 0.5 mL/min. Quantification was carried out using triple quadrupole mass detection with electrospray ionization (ESI) in a multiple reaction monitoring technique. The finalized method was validated successfully, affording ICH guidelines. All N-nitroso impurities revealed excellent linearity over the concentration range of 0.00125-0.0250 ppm. The Pearson correlation coefficient of each N-nitroso impurity was >0.999. The method accuracy recoveries ranged from 93.98 to 108.08% for the aforementioned N-nitrosamine impurities. Furthermore, the method was effectively applied to quantify N-nitrosamine impurities simultaneously in commercially available formulated samples, with its efficiency recurring at trace levels. Thus, the current method is capable of determining the trace levels of three N-nitroso ciprofloxacin impurities simultaneously from the marketed tablet dosage forms for commercial release and stability testing.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
A Multi-Analyte LC-MS/MS Method for Determination and Quantification of Six Nitrosamine Impurities in Sartans like Azilsartan, Valsartan, Telmisartan, Olmesartan, Losartan and Irbesartan.J Chromatogr Sci. 2024 Feb 2;62(2):147-167. doi: 10.1093/chromsci/bmac059. J Chromatogr Sci. 2024. PMID: 35830866
-
Ultrasensitive LC-MS/MS quantitation of the N-nitroso-dabigatran etexilate impurity in dabigatran etexilate mesylate using an electrospray ionization technique.Anal Methods. 2025 Feb 20;17(8):1797-1803. doi: 10.1039/d4ay01710b. Anal Methods. 2025. PMID: 39887346
-
A sensitive UPLC-MS/MS method for the simultaneous assay and trace level genotoxic impurities quantification of SARS-CoV-2 inhibitor-Molnupiravir in its pure and formulation dosage forms using fractional factorial design.Results Chem. 2023 Dec;6:101019. doi: 10.1016/j.rechem.2023.101019. Epub 2023 Jun 27. Results Chem. 2023. PMID: 37396150 Free PMC article.
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
-
Development and validation of an analytical method for trace-level quantification of genotoxic nitrosamine impurities in losartan and hydrochlorothiazide fixed-dose combination tablets using ultra performance liquid chromatography triple quadrupole mass spectrometry.Rapid Commun Mass Spectrom. 2023 Apr 30;37(8):e9488. doi: 10.1002/rcm.9488. Rapid Commun Mass Spectrom. 2023. PMID: 36740827 Review.
Cited by
-
Nitrosamine Drug Substance-Related Impurities (NDSRIs) in Pharmaceuticals: Formation, Mitigation Strategies, and Emphasis on Mutagenicity Risks.Pharm Res. 2025 Apr;42(4):547-578. doi: 10.1007/s11095-025-03857-9. Epub 2025 Apr 23. Pharm Res. 2025. PMID: 40268857 Review.
-
An update on latest regulatory guidelines and analytical methodologies for N-nitrosamine impurities in pharmaceutical products - 2024.Med Gas Res. 2025 Dec 1;15(4):535-543. doi: 10.4103/mgr.MEDGASRES-D-24-00124. Epub 2025 Apr 29. Med Gas Res. 2025. PMID: 40300889 Free PMC article. Review.
-
A novel LC-TQ-MS/MS method for quantifying mefenamic acid-NDSRI (N-nitroso drug substance-related impurity) in mefenamic acid tablet and pediatric suspension dosage forms: a comparative study with a cost-effective white, green, and blue UPLC method.RSC Adv. 2025 Jan 21;15(3):1957-1969. doi: 10.1039/d4ra08425j. eCollection 2025 Jan 16. RSC Adv. 2025. PMID: 39839227 Free PMC article.
References
-
- 2023https://pubchem.ncbi.nlm.nih.gov/compound/ciprofloxacin#section=NCI-Thes... (accessed May 25).
-
- Shariati A.; Arshadi M.; Khosrojerdi M. A.; Abedinzadeh M.; Ganjalishahi M.; Maleki A.; Heidary M.; Khoshnood S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public. Health. 2022, 10, 102563310.3389/fpubh.2022.1025633. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
