Structural Insights and Influence of Terahertz Waves in Midinfrared Region on Kv1.2 Channel Selectivity Filter
- PMID: 38434859
- PMCID: PMC10905694
- DOI: 10.1021/acsomega.3c09801
Structural Insights and Influence of Terahertz Waves in Midinfrared Region on Kv1.2 Channel Selectivity Filter
Abstract
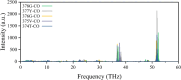
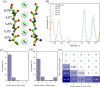
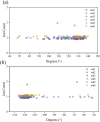
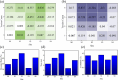
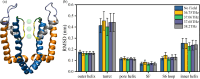
Potassium ion channels are the structural basis for excitation transmission, heartbeat, and other biological processes. The selectivity filter is a critical structural component of potassium ion channels, whose structure is crucial to realizing their function. As biomolecules vibrate and rotate at frequencies in the terahertz band, potassium ion channels are sensitive to terahertz waves. Therefore, it is worthwhile to investigate how the terahertz wave influences the selectivity filter of the potassium channels. In this study, we investigate the structure of the selectivity filter of Kv1.2 potassium ion channels using molecular dynamics simulations. The effect of an electric field on the channel has been examined at four different resonant frequencies of the carbonyl group in SF: 36.75 37.06, 37.68, and 38.2 THz. As indicated by the results, 376GLY appears to be the critical residue in the selectivity filter of the Kv1.2 channel. Its dihedral angle torsion is detrimental to the channel structural stability and the transmembrane movement of potassium ions. 36.75 THz is the resonance frequency of the carbonyl group of 376GLY. Among all four frequencies explored, the applied terahertz electric field of this frequency has the most significant impact on the channel structure, negatively impacting the channel stability and reducing the ion permeability by 20.2% compared to the absence of fields. In this study, we simulate that terahertz waves in the mid-infrared frequency region can significantly alter the structure and function of potassium ion channels and that the effects of terahertz waves differ greatly based on frequency.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Permeability enhancement of Kv1.2 potassium channel by a terahertz electromagnetic field.J Chem Phys. 2023 Jul 28;159(4):045101. doi: 10.1063/5.0143648. J Chem Phys. 2023. PMID: 37486058
-
Effect of Terahertz Electromagnetic Field on the Permeability of Potassium Channel Kv1.2.Int J Mol Sci. 2023 Jun 17;24(12):10271. doi: 10.3390/ijms241210271. Int J Mol Sci. 2023. PMID: 37373419 Free PMC article.
-
Terahertz waves promote Ca2+ transport in the Cav2.1 channel.Spectrochim Acta A Mol Biomol Spectrosc. 2025 Aug 5;336:126039. doi: 10.1016/j.saa.2025.126039. Epub 2025 Mar 11. Spectrochim Acta A Mol Biomol Spectrosc. 2025. PMID: 40112754
-
Structure of the voltage-gated potassium channel KV1.3: Insights into the inactivated conformation and binding to therapeutic leads.Channels (Austin). 2023 Dec;17(1):2253104. doi: 10.1080/19336950.2023.2253104. Channels (Austin). 2023. PMID: 37695839 Free PMC article. Review.
-
Ion-protein interactions of a potassium ion channel studied by attenuated total reflection Fourier transform infrared spectroscopy.Biophys Rev. 2018 Apr;10(2):235-239. doi: 10.1007/s12551-017-0337-8. Epub 2017 Nov 22. Biophys Rev. 2018. PMID: 29168118 Free PMC article. Review.
Cited by
-
Ultrafast terahertz-induced torque disruption of fentanyl's μ-opioid receptor binding for precision overdose reversal.J Mol Model. 2025 Jul 29;31(8):221. doi: 10.1007/s00894-025-06447-z. J Mol Model. 2025. PMID: 40728567
-
A Non-Invasive and DNA-free Approach to Upregulate Mammalian Voltage-Gated Calcium Channels and Neuronal Calcium Signaling via Terahertz Stimulation.Adv Sci (Weinh). 2024 Dec;11(47):e2405436. doi: 10.1002/advs.202405436. Epub 2024 Oct 22. Adv Sci (Weinh). 2024. PMID: 39435751 Free PMC article.
-
Impact of a Terahertz electromagnetic field on the ion permeation of potassium and sodium channels.Commun Chem. 2025 Apr 3;8(1):101. doi: 10.1038/s42004-025-01503-4. Commun Chem. 2025. PMID: 40175698 Free PMC article.
-
Terahertz Science and Technology in Astronomy, Telecommunications, and Biophysics.Research (Wash D C). 2025 Jan 22;8:0586. doi: 10.34133/research.0586. eCollection 2025. Research (Wash D C). 2025. PMID: 39845706 Free PMC article. Review.
References
-
- Hille B.Ion Channels of Excitable Membranes; Sinauer Associates: Sunderland, Massachusetts U.S.A., 2001.
-
- Wu X.; Jiang Y.; Rommelfanger N. J.; Yang F.; Zhou Q.; Yin R.; Liu J.; Cai S.; Ren W.; Shin A.; et al. Tether-Free Photothermal Deep-Brain Stimulation in Freely Behaving Mice via Wide-Field Illumination in the Near-Infrared-II Window. Nat. Biomed. Eng. 2022, 6, 754–770. 10.1038/s41551-022-00862-w. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
